4120
Multiparametric MRI for Staging of Bowel Inflammatory Activity in Crohn's Disease with IVIM and DCE-MRI: A Preliminary Study1Radiology, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China, 2MR Research China, GE Healthcare, Beijing, China
Synopsis
Keywords: Digestive, Diffusion/other diffusion imaging techniques
In summary, our findings suggested that the combination of IVIM and DCE-MRI can be used to accurately stage CD lesions activity. However, we must acknowledge that further investigation area is warranted to validate this preliminary finding.Background:
In Crohn's disease (CD) clinical practice, an accurate staging of inflammatory activity is more importantant than detecting lesions, as clinical management decision and efficacy evaluation are dependent on activity stage.Purpose:
To investigate if the combination of intravoxel incoherent motion (IVIM) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is feasible for staging CD activity.Study Type:
Retrospective.Population:
A total of 65 CD patients (45 men and 20 women; age range 18~65 years, median age 29.78 years) were enrolled and analyzed.Field Strength/Sequence:
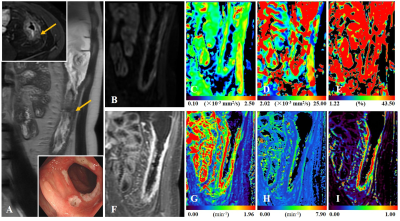
A 3.0-T, T1-weighted, T2-weighted, IVIM (multiplexed sensitivity encoding, MUSE), DCE images.Assessment:
The simplified endoscopic score for Crohn’s Disease (SES-CD) and magnetic resonance index of activity (MaRIA) were used as the reference. The severity and extent of bowel lesions were evaluated by a gastroenterologist using standard colonoscopy. A senior radiologist scored the ileocolic lesions using the MaRIA scoring system. The IVIM and DCE-MRI data were processed by two junior radiologists, who were blinded to the SES-CD and MaRIA scores. Finally, three IVIM parameters: fast apparent diffusion coefficient (ADCfast), slow apparent diffusion coefficient (ADCslow), and the fractional perfusion (Fraction of ADCfast), as well as four DCE-MRI parameters: volume transfer constant (Ktrans), rate constant (Kep), extravascular extracellular volume fraction (Ve) and plasma volume fraction (Vp) were generated.Statistical Tests:
Intra-class correlation coefficient (ICC), non-parametric test (Kruskal-Wallis H and Mann-Whitney U), logistic regression, receiver operating characteristic (ROC) analysis, Delong test, and Spearman's correlation test were performed.Results:
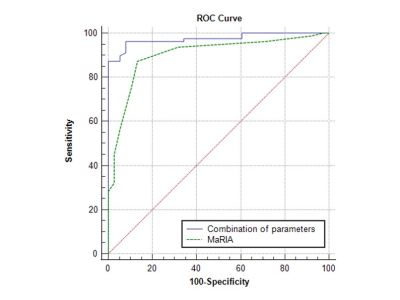
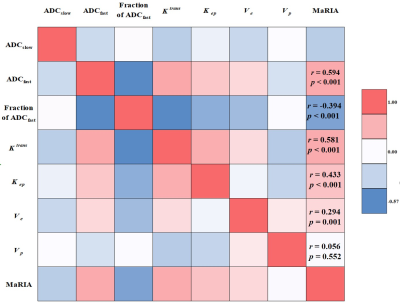
According to SES-CD, 116 ileocolonic segments with CD lesions were indentified as: inactive (n = 38), mild (n = 46), and moderate to severe (n = 32). Inter-reader agreement has been validated with high ICC coefficients > 0.9 for all IVIM and DCE-MRI parameters. Relative to the two active groups, MaRIA (6, p < 0.001), ADCfast (8.68 ×10-3 mm2/s, p < 0.001), Ktrans (0.95 min-1, p < 0.001), Kep (1.14 min-1, p < 0.001) and Ve (0.79, p < 0.001) all showed lower values in inactive group. Fraction of ADCfast (38.8%, p < 0.001) was higher in inactive than the other two groups. With multivariable logistic regression analysis, ADCfast (p < 0.001), Fraction of ADCfast (p = 0.005), Ktrans (p < 0.001) and Kep (p = 0.003) were identified as significant factors for differentiating among the three groups. Significantly different parameters were revealed between inactive and active groups, including MaRIA score (6 vs. 12.5, p < 0.001), ADCfast (8.68×10-3 mm2/s vs. 11.89×10-3 mm2/s, p < 0.001), Fraction of ADCfast (38.80% vs. 25.63%, p < 0.001), Ktrans (0.95 min-1 vs. 1.82 min-1, p < 0.001), Kep (1.14 min-1 vs. 1.64 min-1, p < 0.001) and Ve (0.79 vs. 0.87, p = 0.011). Binary logistic analyses identified ADCfast (Odds Ratio = 2.009, p = 0.001), Ktrans (Odds Ratio = 54.771, p = 0.014) and Kep (Odds Ratio = 6.105, p = 0.029) as independent predictors for the active status. The combination of ADCfast, Ktrans and Kep performed better than MaRIA score (p = 0.028), for differentiating inactive and active status. MaRIA score was positively correlated with ADCfast (r = 0.594, p < 0.001), Ktrans (r = 0.581, p < 0.001), Kep (r = 0.443, p < 0.001) and Ve (r = 0.294, p = 0.001), however, negatively correlated with Fraction of ADCfast (r = -0.394, p < 0.001).Data Conclusion:
The combination of IVIM and DCE-MRI can be used to accurately stage inflammatory activity in CD.Acknowledgements
The research project was supported by the “Six One Projects” Top-notch Talent Research Project for High-level Health Talents in Jiangsu Province, China (Grant No. LGY2018072).References
1. Roda G, Chien Ng S, Kotze PG, et al. Crohn's disease. Nat Rev Dis Primers 2020;6(1):22.
2. Cushing K, Higgins PDR. Management of Crohn Disease: A Review. JAMA 2021;325(1):69-80.
3. Bharadwaj S, Narula N, Tandon P, Yaghoobi M. Role of endoscopy in inflammatory bowel disease. Gastroenterol Rep (Oxf) 2018;6(2):75-82.
4. Chatterji M, Fidler JL, Taylor SA, Anupindi SA, Yeh BM, Guglielmo FF. State of the Art MR Enterography Technique. Top Magn Reson Imaging 2021;30(1):3-11.
5. Rimola J, Ordas I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis 2011;17(8):1759-1768.
6. Somwaru AS, Khanijow V, Katabathina VS. Magnetic resonance enterography, colonoscopy, and fecal calprotectin correlate in colonic Crohn's disease. BMC Gastroenterol 2019;19(1):210.
7. Lee S, Choi YH, Cho YJ, et al. Quantitative evaluation of Crohn's disease using dynamic contrast-enhanced MRI in children and young adults. Eur Radiol 2020;30(6):3168-3177.
8. Zhu J, Zhang F, Luan Y, et al. Can Dynamic Contrast-Enhanced MRI (DCE-MRI) and Diffusion-Weighted MRI (DW-MRI) Evaluate Inflammation Disease: A Preliminary Study of Crohn's Disease. Medicine (Baltimore) 2016;95(14): e3239.
9. Zhu J, Zhang F, Zhou J, Li H. Assessment of therapeutic response in Crohn's disease using quantitative dynamic contrast enhanced MRI (DCE-MRI) parameters: A preliminary study. Medicine (Baltimore) 2017;96(32): e7759.
10. Bhatnagar G, Dikaios N, Prezzi D, Vega R, Halligan S, Taylor SA. Changes in dynamic contrast-enhanced pharmacokinetic and diffusion-weighted imaging parameters reflect response to anti-TNF therapy in Crohn's disease. Br J Radiol 2015;88(1055):20150547.
11. Federau C. Measuring Perfusion: Intravoxel Incoherent Motion MR Imaging. Magn Reson Imaging Clin N Am 2021;29(2):233-242.
12. Freiman M, Perez-Rossello JM, Callahan MJ, et al. Characterization of fast and slow diffusion from diffusion-weighted MRI of pediatric Crohn's disease. J Magn Reson Imaging 2013;37(1):156-163.
13. Le Bihan D. Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology 2008;249(3):748-752.
14. Hectors SJ, Gordic S, Semaan S, et al. Diffusion and perfusion MRI quantification in ileal Crohn's disease. European radiology 2019;29(2):993-1002.
15. Lefrancois P, Zummo-Soucy M, Olivie D, et al. Diagnostic performance of intravoxel incoherent motion diffusion-weighted imaging and dynamic contrast-enhanced MRI for assessment of anal fistula activity. PLoS One 2018;13(1): e0191822.
16. Qin J, Li J, Yang H, et al. Values of intravoxel incoherent motion diffusion weighted imaging and dynamic contrast-enhanced MRI in evaluating the activity of sacroiliitis in ankylosing spondylitis of rat model. Magn Reson Imaging 2020; 68:30-35.
17. Zou L, Jiang J, Zhang H, et al. Comparing and combining MRE, T1rho, SWI, IVIM, and DCE-MRI for the staging of liver fibrosis in rabbits: Assessment of a predictive model based on multiparametric MRI. Magn Reson Med 2022;87(5):2424-2435.
18. Andrade AR, da Rocha TRF, Ortiz-Agostinho CL, et al. Endoscopic activity, tissue factor and Crohn's disease: findings in clinical remission patients. Therap Adv Gastroenterol 2020; 13:1756284820939412.
19. Roseira J, Ventosa AR, de Sousa HT, Brito J. The new simplified MARIA score applies beyond clinical trials: A suitable clinical practice tool for Crohn's disease that parallels a simple endoscopic index and fecal calprotectin. United European Gastroenterol J 2020;8(10):1208-1216.
20. Weiss B, Turner D, Griffiths A, et al. Simple Endoscopic Score of Crohn Disease and Magnetic Resonance Enterography in Children: Report from ImageKids Study. J Pediatr Gastroenterol Nutr 2019;69(4):461-465.
21. Yang X, Xiao X, Lu B, Chen Y, Wen Z, Yu S. Perfusion-sensitive parameters of intravoxel incoherent motion MRI in rectal cancer: evaluation of reproducibility and correlation with dynamic contrast-enhanced MRI. Acta Radiol 2019;60(5):569-577.
22. Sun H, Xu Y, Xu Q, et al. Correlation Between Intravoxel Incoherent Motion and Dynamic Contrast-Enhanced Magnetic Resonance Imaging Parameters in Rectal Cancer. Acad Radiol 2019;26(7): e134-e140.
23. Rozendorn N, Amitai MM, Eliakim RA, Kopylov U, Klang E. A review of magnetic resonance enterography-based indices for quantification of Crohn's disease inflammation. Therap Adv Gastroenterol 2018; 11:1756284818765956.
24. D'Amico F, Chateau T, Laurent V, Danese S, Peyrin-Biroulet L. Which MRI Score and Technique Should Be Used for Assessing Crohn's Disease Activity? J Clin Med 2020;9(6).
25. Zhang MC, Li XH, Huang SY, et al. IVIM with fractional perfusion as a novel biomarker for detecting and grading intestinal fibrosis in Crohn's disease. Eur Radiol 2019;29(6):3069-3078.
26. Wu YC, Xiao ZB, Lin XH, Zheng XY, Cao DR, Zhang ZS. Dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging in the activity staging of terminal ileum Crohn's disease. World J Gastroenterol 2020;26(39):6057-6073.
27. Vieujean S, Coibion C, Seidel L, Louis E, Meunier P. Magnetic resonance enterography perfusion parameters reveal complex changes in affected and unaffected segments in Crohn's disease. Scand J Gastroenterol 2020;55(9):1041-1048.
28. Baxter GC, Patterson AJ, Woitek R, Allajbeu I, Graves MJ, Gilbert F. Improving the image quality of DWI in breast cancer: comparison of multi-shot DWI using multiplexed sensitivity encoding to conventional single-shot echo-planar imaging DWI. Br J Radiol 2021;94(1119):20200427.
29. Oto A, Kayhan A, Williams JT, et al. Active Crohn's disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 2011;33(3):615-624.
30. Eder P, Lykowska-Szuber L, Iwanik K, et al. The influence of anti-TNF therapy on CD31 and VEGF expression in colonic mucosa of Crohn's disease patients in relation to mucosal healing. Folia Histochem Cytobiol 2016;54(2):75-80.
31. Song XL, Wang L, Ren H, Wei R, Ren JL, Niu J. Intravoxel Incoherent Motion Imaging in Differentiation Borderline from Malignant Ovarian Epithelial Tumors: Correlation with Histological Cell Proliferation and Vessel Characteristics. J Magn Reson Imaging 2020;51(3):928-935.
32. Deban L, Correale C, Vetrano S, Malesci A, Danese S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. Am J Pathol 2008;172(6):1457-1466.
Figures