4113
Value of BOLD MR in early evaluating the response and prognosis of esophageal squamous cell carcinoma treated with definitive chemoradiotherapy1Department of Radiology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China, 2The Comprehensive Cancer Center of Drum Tower Hospital, Medical School of Nanjing University and Clinical Cancer Institute of Nanjing University, Nanjing, China
Synopsis
Keywords: Digestive, fMRI
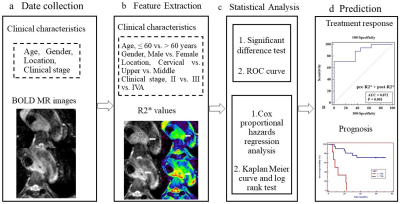
To confirm a quantitative imaging predictor for evaluation of early treatment response and prognosis to definitive chemoradiotherapy (dCRT) in patients with esophageal squamous cell carcinoma , using BOLD MR images. R2* values were obtained pre- and post-dCRT in 28 patients using BOLD MR. Independent samples t-test (normality) or Mann-Whitney U test (non-normality) was used to compare differences of R2*-related parameters between CR and non-CR groups. Diagnostic performance of parameters in predicting response was tested with receiver operating characteristic curve analysis. 3-years overall survival (OS) was evaluated using by Kaplan Meier curve, log rank test, and Cox proportional hazards regression analysis.Purpose
To confirm a quantitative imaging predictor for evaluation of early treatment response and prognosis to definitive chemoradiotherapy (dCRT) in patients with esophageal squamous cell carcinoma (ESCC), using blood oxygenation level-dependent (BOLD) magnetic resonance (MR) images.Methods:
R2* values were obtained pre- and 2-3 weeks post-dCRT in 28 patients with EC using BOLD MR. Independent samples t-test (normality) or Mann-Whitney U test (non-normality) was used to compare differences of R2*-related parameters between complete response (CR) and non-CR groups. Diagnostic performance of parameters in predicting response was tested with receiver operating characteristic curve analysis. 3-years overall survival (OS) was evaluated using by Kaplan Meier curve, log rank test, and Cox proportional hazards regression analysis.Results
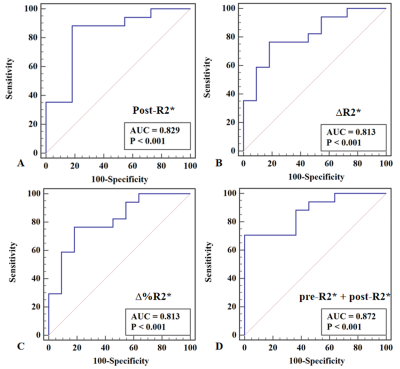
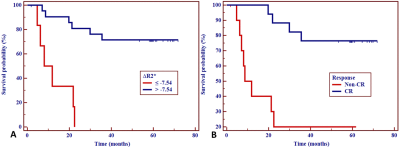
Post-R2*, ∆R2*, and ∆%R2* in CR group were significantly higher than those in non-CR group (P = 0.002, 0.003, and 0.006, respectively). The R2*-related parameters showed good prediction of tumor response with AUC ranging from 0.813 to 0.872. 3-year OS rate in patients with ∆R2* >-7.54 s-1 or a CR were significantly longer than those with ∆R2* ≤ -7.54 s-1 (72.37% vs. 0.00%; Hazard ratio, HR = 0.196; 95% confidence interval, 95%CI = 0.047-0.807; P = 0.024) or non-CR (76.47% vs. 29.27%; HR = 0.238, 95%CI = 0.059-0.963; P = 0.044).Conclusion
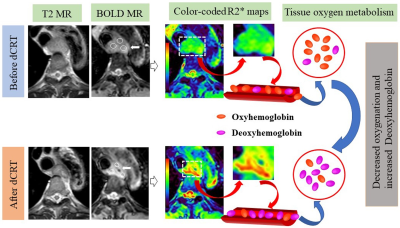
Our preliminary results demonstrated that the R2* value might be a useful hypoxia imaging predictor for response and prognosis of ESCC treated with dCRT. BOLD MR imaging might be used as a potential tool for evaluating tumor oxygenation metabolism, which is routinely applied in clinical practice and beneficial to clinical decision-making.Keywords
Esophageal neoplasms; Blood oxygenation level-dependent; Response; Prognosis; ChemoradiotherapyAcknowledgements
This study received funding from the Nanjing Drum Tower Hospital New Technology Development Fund (XJSFZJJ202035); Bethune·Young and Middle-aged Physician Scientific Research Ability Training Project (BQE-TY-SSPC(7)-N-01); Special Fund for Clinical Scientific Research of Wu Jieping Medical Foundation (320.6750.2021-01-36).References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 71(3) (2021) 209-249. doi: 10.3322/caac.21660.
2. Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 25 (10) (2007) 1160-8. doi: 10.1200/JCO.2005.04.7118.
3. Teoh AY, Chiu PW, Yeung WK, Liu SY, Wong SK, Ng EK. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol. 24 (1) (2013) 165-71. doi: 10.1093/annonc/mds206.
4. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 3 (2015) 83-92. doi: 10.2147/HP.S93413.
5. Lin Q, Yun Z. Impact of the hypoxic tumor microenvironment on the regulation of cancer stem cell characteristics. Cancer Biol Ther. 9(12) (2010) 949-56. doi: 10.4161/cbt.9.12.12347.
6. Yue J, Yang Y, Cabrera AR, Sun X, Zhao S, Xie P, Zheng J, Ma L, Fu Z, Yu J. Measuring tumor hypoxia with ¹⁸F-FETNIM PET in esophageal squamous cell carcinoma: a pilot clinical study. Dis Esophagus. 25 (1) (2012) 54-61. doi: 10.1111/j.1442-2050.2011.01209.x.
7. Rich LJ, Seshadri M. Photoacoustic imaging of vascular hemodynamics: validation with blood oxygenation level-dependent MR imaging. Radiology. 275 (1) (2015) 110-8. doi: 10.1148/radiol.14140654.
8. Liu P, Liu G, Pinho MC, Lin Z, Thomas BP, Rundle M, Park DC, Huang J, Welch BG, Lu H. Cerebrovascular Reactivity Mapping Using Resting-State BOLD Functional MRI in Healthy Adults and Patients with Moyamoya Disease. Radiology. 299 (2) (2021) 419-425. doi: 10.1148/radiol.2021203568.
9. Huang YL, Zhou JL, Jiang YM, Zhang ZG, Zhao W, Han D, He B. Assessment of lumbar paraspinal muscle activation using fMRI BOLD imaging and T2 mapping. Quant Imaging Med Surg. 10 (1) (2020)106-115. doi: 10.21037/qims.2019.10.20.
10. Caroca S, Villagran D, Chabert S. Four functional magnetic resonance imaging techniques for skeletal muscle exploration, a systematic review. Eur J Radiol. 144 (2021) 109995. doi: 10.1016/j.ejrad.2021.109995.
11. Chang D, Wang YC, Xu TT, Peng XG, Cai Y, Wang L, Bai YY, Ju S. Noninvasive Identification of Renal Hypoxia in Experimental Myocardial Infarctions of Different Sizes by Using BOLD MR Imaging in a Mouse Model. Radiology. 286 (1) (2018) 129-139. doi: 10.1148/radiol.2017161998.
12. Chaudhry AA, Naim S, Gul M, Chaudhry A, Chen M, Jandial R, Badie B. Utility of Preoperative Blood-Oxygen-Level-Dependent Functional MR Imaging in Patients with a Central Nervous System Neoplasm. Radiol Clin North Am. 57 (6) (2019) 1189-1198. doi: 10.1016/j.rcl.2019.07.006.
13. Fusco R, Granata V, Pariante P, Cerciello V, Siani C, Di Bonito M, Valentino M, Sansone M, Botti G, Petrillo A. Blood oxygenation level dependent magnetic resonance imaging and diffusion weighted MRI imaging for benign and malignant breast cancer discrimination. Magn Reson Imaging. 75 (2021) 51-59. doi: 10.1016/j.mri.2020.10.008.
14. Miyata M, Aoki T, Shimajiri S, Matsuyama A, Kinoshita S, Fujii M, Katsuki T, Inoue Y, Nagata Y, Tashima Y, Korogi Y. Evaluation of the R2* value in invasive ductal carcinoma with respect to hypoxic-related prognostic factors using iterative decomposition of water and fat with echo asymmetry and least-squares emission (IDEAL). Eur Radiol. 27 (10) (2017) 4316-4323. doi: 10.1007/s00330-017-4832-x.
15. Peng Y, Luo Y, Hu X, Shen Y, Hu D, Li Z, Kamel I. Quantitative T2*-Weighted Imaging and Reduced Field-of-View Diffusion-Weighted Imaging of Rectal Cancer: Correlation of R2* and Apparent Diffusion Coefficient With Histopathological Prognostic Factors. Front Oncol. 11(2021) 670156. doi: 10.3389/fonc.2021.670156.
16. Alonzi R, Padhani AR, Maxwell RJ, Taylor NJ, Stirling JJ, Wilson JI, d'Arcy JA, Collins DJ, Saunders MI, Hoskin PJ. Carbogen breathing increases prostate cancer oxygenation: a translational MRI study in murine xenografts and humans. Br J Cancer. 100 (4) (2009) 644-8. doi: 10.1038/sj.bjc.6604903.
17. Kim CK, Park SY, Park BK, Park W, Huh SJ. Blood oxygenation level-dependent MR imaging as a predictor of therapeutic response to concurrent chemoradiotherapy in cervical cancer: a preliminary experience. Eur Radiol. 24 (7) (2014)1514-20. doi: 10.1007/s00330-014-3167-0.
18. Lee J, Kim CK, Gu KW, Park W. Value of blood oxygenation level-dependent MRI for predicting clinical outcomes in uterine cervical cancer treated with concurrent chemoradiotherapy. Eur Radiol. 29 (11) (2019) 6256-6265. doi: 10.1007/s00330-019-06198-5.
19. Li SP, Taylor NJ, Makris A, Ah-See ML, Beresford MJ, Stirling JJ, d'Arcy JA, Collins DJ, Padhani AR. Primary human breast adenocarcinoma: imaging and histologic correlates of intrinsic susceptibility-weighted MR imaging before and during chemotherapy. Radiology. 257 (3) (2010) 643-52. doi: 10.1148/radiol.10100421.
20. Tang YL, Zhang XM, Yang ZG, Huang YC, Chen TW, Chen YL, Chen F, Zeng NL, Li R, Hu J. The Blood Oxygenation T2* Values of Resectable Esophageal Squamous Cell Carcinomas as Measured by 3T Magnetic Resonance Imaging: Association with Tumor Stage. Korean J Radiol. 18 (4) (2017) 674-681. doi: 10.3348/kjr.2017.18.4.674.
21. Riddell AM, Allum WH, Thompson JN, Wotherspoon AC, Richardson C, Brown G. The appearances of oesophageal carcinoma demonstrated on high-resolution, T2-weighted MRI, with histopathological correlation. Eur Radiol. 17 (2) (2007) 391-9. doi: 10.1007/s00330-006-0363-6.
22. Foti PV, Privitera G, Piana S, Palmucci S, Spatola C, Bevilacqua R, Raffaele L, Salamone V, Caltabiano R, Magro G, Li Destri G, Milone P, Ettorre GC. Locally advanced rectal cancer: Qualitative and quantitative evaluation of diffusion-weighted MR imaging in the response assessment after neoadjuvant chemo-radiotherapy. Eur J Radiol Open. 3 (2016) 145-52. doi: 10.1016/j.ejro.2016.06.003.
23. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 10 (21) (2004) 7252-9. doi: 10.1158/1078-0432.
24. Sun NN, Liu C, Ge XL, Wang J. Dynamic contrast-enhanced MRI for advanced esophageal cancer response assessment after concurrent chemoradiotherapy. Diagn Interv Radiol. 24 (4) (2018) 195-202. doi: 10.5152/dir.2018.17369.
25. Li XS, Fan HX, Fang H, Song YL, Zhou CW. Value of R2* obtained from T2*-weighted imaging in predicting the prognosis of advanced cervical squamous carcinoma treated with concurrent chemoradiotherapy. J Magn Reson Imaging. 42 (3) (2015) 681-8. doi: 10.1002/jmri.24837.
26. Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 23(4) (2013) 233-42. doi: 10.2188/jea.je20120162.
Figures