4104
Highly accelerated 3D hyperpolarized MRI at ultra-high fields using Seiffert Spirals1Internal Medicine II, Ulm University Medical Center, Ulm, Germany, 2NVision Imaging Technologies GmbH, Ulm, Germany
Synopsis
Keywords: New Trajectories & Spatial Encoding Methods, Data Acquisition
Isotropic 3D imaging of hyperpolarized media is generally challenging due to the extremely limited lifetime of the hyperpolarized phase. Fortunately, images of hyperpolarized media are often intrinsically sparse, resulting in an excellent precondition for non-linear reconstruction techniques such as Compressed Sensing. To preserve this condition, a 3D Seiffert Spiral trajectory was implemented at 11.7 T, yielding low-coherent sampling properties for an arbitrary number of excitations, until depletion of the hyperpolarized signal. We show that qualitative images with a resolution of <500 $$$\mu$$$m can be reconstructed from continuous data that was acquired in less than one second.Introduction
Various studies have focused on hyperpolarized [1-13C]pyruvate by observing its uptake and conversion to [1-13C]lactate, [1-13C]alanine, 13C-bicarbonate, and 13CO2. These studies have shown tremendous potential for metabolic imaging of cardiac diseases1,2 and cancer3,4. Hyperpolarized cancer studies have e.g. demonstrated that the lactate dehydrogenase-catalyzed reaction involving pyruvate and lactate results in dramatically higher levels of hyperpolarized 13C lactate in tumours than in normal tissue.The localization of regions of hyperpolarized agent accumulation makes 3D imaging more appealing, but its associated scan times due to the required number of excitations for Nyquist sampling often impedes its application.
The previously introduced 3D Seiffert Spirals5,6 have the advantageous property of low-coherent sampling for an arbitrary number of interleaves, making it a perfect trajectory for intrinsically sparse signals that are not disturbed by off-resonances. Furthermore, Seiffert Spirals only consider the resolution as a scan parameter and any FOV might be reconstructed from data that was acquired for as long as the lifetime of the hyperpolarized phase persists. This concept was already demonstrated for renal perfusion imaging at a clinical 3 T MRI system7.
Methods
The general waveform of one interleave was generated on the surface of a unit sphere according to the definition:$$$\zeta: \mathbb{R}_0^+ \rightarrow \mathbb{R}^3$$$, $$$s \mapsto \zeta(s)$$$ with $$$m\in (0,1)$$$ and
$$\begin{align}\zeta_x(s,m) &= \theta_1(s,m^2)\cdot \cos(s\cdot m^2)\\\zeta_y(s,m) &= \theta_1(s,m^2)\cdot \sin(s\cdot m^2)\\\zeta_z(s,m) &= \theta_2(s,m^2)\end{align}$$
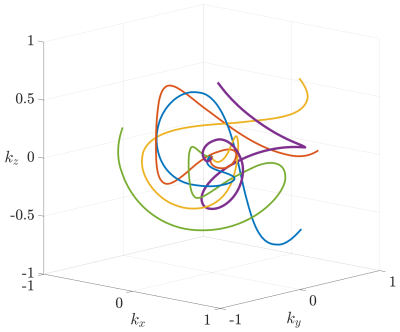
with $$$\theta_1$$$ and $$$\theta_2$$$ being the first two Jacobi theta functions. Furthermore, $$$m$$$ was chosen such that hardware limitations (maximum slew-rate and gradient amplitudes) were not violated during the read-out duration of 1.5 ms. Figure 1 shows five rotated interleaves with each end-point being a Fibonacci point on the surface of the k-space sphere.
Phantom measurements were performed on a 11.7 T pre-clinical MRI system (BioSpec 117/16 USR, Bruker, Ettlingen, Germany) using a quadrature and dual tuned 13C/1H coil with an inner diameter of 30 mm (Rapid Biomedical GmbH, Rimpar, Germany).
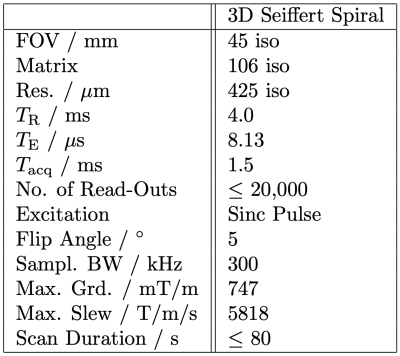
Hyperpolarized pyruvate was generated by hydrogenation of a 13C labelled precursor with para-enriched hydrogen. The polarization of the proton singlet state was converted into 13C magnetization using a linear magnetic field sweep. After detoxification, 400 $$$\mu$$$l of the hyperpolarized 13C pyruvate solution were injected into the phantom (medical tubing within copper sulfate-doped water) and directly imaged using 3D Seiffert Spirals with scan parameters shown in figure 2, until depletion of the hyperpolarized signal.
13C reference power calibration was achieved using a dedicated 13C-enriched urea phantom.
Results
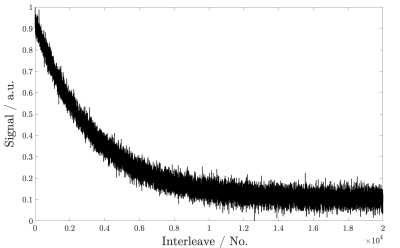
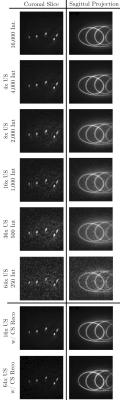
Figure 3 shows the exponential decay of the hyperpolarized 13C signal during imaging. The signal corresponds to the normalised signal amplitude of the first acquired k-space point of each interleave. Considering the signal evolution, the first 16,000 interleaves were used for image reconstruction and retrospectively undersampled based on relative undersampling factors 4, 8, 16, 32 and 64. Undersampled data was chosen throughout the entire acquisition and not just from early (high SNR) interleaves. All reconstructed images are shown in figure 4 with additional CS reconstructions of datasets of 16-fold and 64-fold undersampling.Figure 5 highlights that the reconstructed sagittal projection is in accordance with acquired 1H images of the imaging phantom. The overlay shows the reconstructed 13C image using a heat colour-map on top of the 1H image, acquired using a 2D FLASH sequence.
Discussion and Conclusion
All presented results indicate low-coherent aliasing properties, leading to a noise-like aliasing behaviour, as previously shown in 5,6,7. Qualitative 3D images were reconstructable from data, acquired in less than one second, in combination with a Compressed Sensing reconstruction, enabling the possibility for time-resolved in-vivo perfusion imaging (sliding-window).The presented trajectory was furthermore generated without consideration of the imaging FOV, by solely focusing on the desired image resolutions, since the approach enables the reconstruction of arbitrary FOVs by only introducing low-coherent aliasing artefacts. This can omit the necessity of 13C scan planing based on 1H survey data which is often difficult to acquire (multi-coil setup).
The application might furthermore enable the use of longer read-outs (higher sampling efficiency) since off-resonance effects appear less deteriorating despite ultra-high field strengths.
The presented trajectory and its properties might furthermore be exploited for 3D chemical shift imaging, based on varying echo times.
Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 858149.
The authors thank the Ulm University Centre for Translational Imaging MoMAN for its support.
References
[1] Golman K, Peterson JS. Metabolic imaging and other applications of hyperpolarized 13C. Acad Radiol. 2006;13:932–942.
[2] Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc Natl Acad Sci USA. 2008;105:12051–12056.
[3] Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006;66:10855–10860.
[4] Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13:1382–1387.
[5] Speidel, T, Metze, P, Rasche, V. (2018). Efficient 3D Low-Discrepancy k-Space Sampling Using Highly Adaptable Seiffert Spirals. IEEE Transactions on Medical Imaging, 38(8), 2018, 1833-1840.
[6] Speidel, T, Metze, P, Stumpf, K, Hüfken, T, Rasche, V. Design and Analysis of Field-of-View Independent k-Space Trajectories for Magnetic Resonance Imaging. Frontiers in Physics, 2022, 504.
[7] Olin, RB, Speidel, T, Sanchez, JD, Hansen, ES, Laustsen, C, Hanson, L G, Ardenkjær-Larsen, JH. Seiffert spirals for hyperpolarized 13C MRI with efficient k-space sampling and flexible acceleration. In Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting.
Figures