4103
Multiple-Slice, Multiple-Breath Washout Hyperpolarized 129Xe Ventilation Mapping of the Lung using Accelerated Data Acquisition1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 3Siemens Healthcare Limited, Montreal, QC, Canada, 4Division of Respiratory Medicine, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
Keywords: Parallel Imaging, Hyperpolarized MR (Gas), multiple-breath washout, multiple-slice, fractional ventilation
Multiple-breath washout hyperpolarized 129Xe MRI (MBW Xe-MRI) results in a regional map of fractional ventilation (FV), measuring percent gas clearance/breath. One limitation of previous work from our group is the single thick coronal slice (200mm) centered on the chest cavity results in significant partial volume effects. Acquiring multiple slices using parallel imaging adds spatial resolution in the slice direction. Feasibility of multiple-slice FV mapping using parallel acceleration is investigated in one adult. Median [IQR] FV was 0.35 [0.30 0.36]. Gravitational dependence was observed in posterior-anterior direction (-0.0122 cm-1). Multiple-slice MBW Xe-MRI is feasible in healthy adults using parallel image acquisition.Introduction
Sensitive tools to monitor changes in early cystic fibrosis (CF) lung disease are needed to assess efficacy of novel therapies. The lung clearance index (LCI), derived from the multiple-breath washout (MBW) test, was observed to capture treatment effects in children with CF1,2. However, LCI represents a whole-lung ventilation average, thereby missing regional information which limits the sensitivity of LCI to treatment response.Performing MRI during multiple washout breaths-holds following a single inhalation of hyperpolarized (HP) 129Xe gas results in a regional map of fractional ventilation (FV), measuring percent gas clearance per breath, similar to LCI8. This MBW Xe-MRI technique can capture regional changes in ventilation, potentially more sensitively than LCI3. One limitation of previous work from our group is the single thick coronal slice (200 mm) centered on the chest cavity which results in significant partial volume effects in the anterior/posterior (AP) direction, confounding the ability of MBW Xe-MRI to distinguish health from CF4.
Acquiring multiple slices adds spatial resolution in the slice direction and potentially improves MBW Xe-MRI’s ability to detect subtle changes in ventilation kinetics. However, this increases scan duration required during washouts beyond reasonable breath-hold durations. This can be addressed by accelerating signal acquisition using parallel multi-channel receivers. HP imaging is well-suited for parallel imaging as no price is paid by reducing scan time (ex. decreased RF depolarization allows for better signal per line of k-space), unlike thermal imaging. In this work, the feasibility of multiple-slice FV mapping using parallel acceleration is investigated. Multiple-slice FV maps are used to explore the gravitational dependence of ventilation in the AP direction.
Methods
In this proof-of-concept work, one healthy 22-year-old female participant was recruited with institutional Research Ethics Board and Health Canada approval.129Xe gas was polarized using a commercial polarizer (Polarean 9810, Durham, NC). MBW Xe-MRI was performed on a 3T MRI system (Prisma, Siemens, Erlangen, Germany) with rigid elliptical birdcage transmitter and flexible 8-channel receive array (Rapid Biomedical, Rimpar, Germany). The dose bag contained a volume of HP 129Xe equal to 10% total lung capacity (TLC) calculated according to height/sex5 and topped with N2 gas to 1L.
The participant was coached through MBW Xe-MRI as described by Couch et al6, yielding 8 images (3 calibration, 5 washouts chosen for SNR>10).
Each image was acquired at peak inhalation using a 2D 129Xe gradient echo (GRE) sequence with parameters: number of slices=10, slice thickness=18 mm, TR=8.7 ms, TE=2.38 ms, FA=2.6°, FOV=480x480 mm, matrix=32x32, bandwidth=100 Hz/pixel. Parallel imaging was used to shorten scan time to 0.2784 s per slice with acceleration factor 2 and reconstructed using GeneRalized Autocalibrating Partial Parallel Acquisition (GRAPPA).
FV was derived for every voxel from the washout signal decay accounting for variable T1 relaxation6,7. The mean of the FV map was used to represent FV for a given slice. For gravitational dependence, the mean FV for each slice was plotted against the distance from the posterior surface.
Results
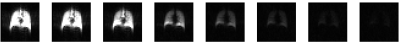
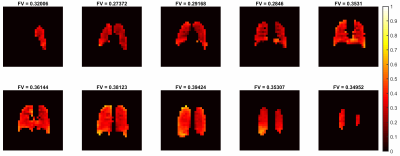
MBW Xe-MRI was well tolerated. Figure 1 shows washout images for the center slice.Figure 2 shows FV maps associated with all 10 slices, anterior (top left) to posterior (bottom right). Median [IQR] FV was 0.35 [0.30 0.36].
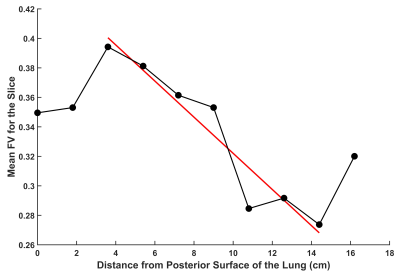
A gravitational dependence of FV was observed in the AP direction (Figure 3), demonstrating a prominent decrease in FV from posterior to anterior (slope=-0.0122 cm-1). Additionally, there was a slight increase in FV observed at the most anterior slice and slight decrease observed at the two most posterior slices.
Discussion
In this preliminary work, parallel imaging acceleration for MBW Xe-MRI was used to acquire more slices within each washout breath-hold. Voxel volumes were significantly improved in this work (4050 mm3) compared to previous single-slice FV maps (11,250 mm3). Due to anticipated signal reductions from the reduced slice thickness, washout images had lower SNR compared to previous single-slice maps (i.e. 4 measurable washout images versus 5-74,6-8). Nevertheless, whole-lung FV values were consistent with single-slice maps previously acquired for healthy adults8, children4, and rats9.The measured gravitational dependence of FV is consistent with studies in healthy rats9 and healthy adults8, with the gradient value -0.0122 cm-1, similar to published values in healthy adults. This dependence is consistent with the known deformation of the lung due to gravity in the supine position, resulting in more efficient washout (i.e. FV) in the dependent lung10. An increase in FV at the posterior surface of the lung and a downturn in FV at the anterior surface of the lung is likely due to some partial volume effects at the edge of the lung with large voxels, and fewer voxels contributing to the gravitational gradient measurement.
Acquiring multiple slices should allow improved detection of gas washout kinetics throughout the entire lung, potentially allowing MBW Xe-MRI to provide a more comprehensive regional interrogation of CF lung disease. An on-going aim is to acquire multiple-slice, multiple-breath images in children, including CF. MBW Xe-MRI may be further improved using fast 3D GRE sequences which allow for volumetric and isotropic coverage of the lung.
Conclusion
Multiple-slice, multiple-breath washout (MBW) Xe-MRI is feasible in healthy adults using parallel image acquisition. This allows investigation of slice-to-slice variations in FV throughout the entire lung.Acknowledgements
This study was funded by the Canadian Institute for Health Research (CIHR) and the Natural Sciences and Engineering Research Council (NSERC). Faiyza Alam was supported by a Restracomp award from the SickKids Research Institute.References
[1] Ratjen
F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, Milla CE, Robinson PD,
Waltz D, Davies JC; VX14-809-109 investigator group. Efficacy and safety
of lumacaftor and ivacaftor in patients aged 6-11 years
with cystic fibrosis homozygous for F508del-CFTR: a randomised,
placebo-controlled phase 3 trial. Lancet
Respir Med. 2017 Jul;5(7):557-567.
[2] Rayment, J. H., Stanojevic,
S., Davis, S. D., Retsch-Bogart, G., & Ratjen, F. (2018). Lung clearance
index to monitor treatment response in pulmonary exacerbations in preschool
children with cystic fibrosis. Thorax, 73(5), 451-458.
doi:10.1136/thoraxjnl-2017-210979
[3] Alam, F., Zanette, B.,
Braganza, S., Jensen, R., Dumas, M., Ratjen, F., & Santyr, G. (2022). Elexacaftor/Tezacaftor/Ivacaftor
Treatment in Pediatric Cystic Fibrosis Lung Disease Reduces Ventilation
Heterogeneity Measured with Hyperpolarized 129Xe Multiple-Breath Washout MRI.
North American Cystic Fibrosis Conference (NACFC).
[4] Alam, F., Zanette, B.,
Braganza, S., Li, D., Ratjen, F., & Santyr, G. (2022). Intra-Visit and
Inter-Visit Repeatability of 129Xe Multiple-Breath Washout MRI in Children with
Stable Cystic Fibrosis Lung Disease. International Society of Magnetic
Resonance in Medicine (ISMRM), 2022.
[5] Thomen,
R., Walkup, L., Roach, D., Cleveland, Z., Clancy, J., & Woods, J. (2017). Hyperpolarized
129Xe for investigation of mild cystic fibrosis lung disease in pediatric
patients. Journal Of Cystic Fibrosis, 16(2), 275-282. doi:
10.1016/j.jcf.2016.07.008
[6] Couch, M., Morgado,
F., Kanhere, N., Kowalik, K., Rayment, J., Ratjen, F., & Santyr, G. (2019).
Assessing the feasibility of hyperpolarized 129Xe multiple‐breath washout MRI
in pediatric cystic fibrosis. Magnetic Resonance In Medicine, 84(1), 304-311.
doi: 10.1002/mrm.28099
[7].
Morgado, F., M. Couch, E. Stirrat, and G. Santyr. Effect of T1 relaxation
on ventilation mapping using hyperpolarized 129Xe
multiple breath wash-out imaging. Magn Reson Med. 80(6):2670-2680
(2018). DOI: 10.1002/mrm.27234
[8] Horn, F.
C., Deppe, M. H., Marshall, H., Parra-Robles, J., & Wild, J. M. (2014).
Quantification of regional fractional ventilation in human subjects by
measurement of hyperpolarized 3he washout with 2D and 3D MRI. Journal of
Applied Physiology, 116(2), 129-139. doi:10.1152/japplphysiol.00378.2013
[9] Couch, M. J., Ouriadov, A., & Santyr, G. E. (2012). Regional
ventilation mapping of the rat lung using hyperpolarized 129xe magnetic
resonance imaging. Magnetic Resonance in Medicine, 68(5), 1623-1631.
doi:10.1002/mrm.24152
[10] Sá, R. C., Cronin, M. V.,
Cortney Henderson, A., Holverda, S., Theilmann, R. J., Arai, T. J., & Kim
Prisk, G. (2010). Vertical distribution of specific ventilation in normal
supine humans measured by oxygen-enhanced Proton MRI. Journal of Applied
Physiology, 109(6), 1950-1959. doi:10.1152/japplphysiol.00220.2010
Figures