4102
Measuring Air Flow Dynamics in the Lungs Using Hyperpolarized 129Xe MRI1University of Pennsylvania, Philadelphia, PA, United States, 2Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 3Boston Children’s Hospital, Boston, MA, United States
Synopsis
Keywords: Data Analysis, Hyperpolarized MR (Gas), Xenon
The ability to non-invasively measure air flow in the lungs could enable the early detection and assessment of abnormal air flow resulting from various obstructive and restrictive diseases. Here, we present a novel method for both obtaining and analyzing ventilation maps using hyperpolarized 129Xe MRI (HXe). In addition to assessing tidal volume and fractional ventilation, we introduce a novel sigmoid function describing inhalation and exhalation that enables the assessment of how fast air flows in the lungs and apply this method in a preclinical model of thoracic insufficiency syndrome (TIS) as well as three human lung transplant (LTx) recipients.
Introduction
Restrictive and obstructive lung diseases are characterized by abnormal air flow into and out of the lungs, increasing the difficulty of respiration due to abnormal airway resistance, lung and chest wall compliance, and ventilation rate. In some diseases like thoracic insufficiency syndrome (TIS), treatment effectiveness can only be evaluated through pulmonary function tests which are difficult to acquire pre-intervention and only provide global measures of lung function. Lung transplant provides a final therapeutic option for end-stage disease, but post-transplant mortality rates remain high due to graft failure, which is still poorly understood. Non-invasive measurements of compromised pulmonary air flow might enable the early detection and assessment of obstructive and restrictive disease states. Here, we developed a dynamic hyperpolarized 129Xe MR imaging technique to measure regional ventilation changes in the lungs, using it to produce dynamic ventilation maps in a preclinical model of thoracic insufficiency syndrome (TIS) as well as three lung transplant (LTX) recipients.Methods
Two healthy subjects, one right-lung unilateral, and two bilateral lung transplant recipients were imaged; in another set of experiments, two Yucatan miniature pigs: one healthy and one underwent rib tethering on the right side (T3-9) at 6 weeks-old (TIS model) were imaged at 5 months-old). All images were acquired on 1.5T Siemens scanner using an 8-channel 129Xe-coil during free breathing. A pneumotach flow sensor and 129Xe injection (about 2.5L) were timed synchronously with inhalation1. 3D-spiral interleaves were acquired continuously over four minutes of imaging with TR/TE = 7.63/0.62 ms, flip angle of 4 ̊, and reconstructed onto 80×80x80 grids with FOV of 150 mm isotropic for pigs and 350 mm for humans. The diaphragm position over time was used to retrospectively bin each image into 16 phases of a representative breathing cycle. Symmetric image normalization (Syn) with cross correlation metric in the ANTs toolbox was used to co-register all frames to the end-inhale frame to allow voxel-wise analysis. Registered voxel regions, nominally corresponding to the same tissue volume at each bin time tn, were then characterized by signal intensities Sn. A model was then fit to Sn to derive the local volumes Vn, subject to the constraints that: 1) local volume increases during inhalation result in the addition of gas with a fixed HP magnetization (S0), 2) new gas mixes with residual gas, 3) volume decrease during exhalation results in signal loss proportional to the fractional reduction in local gas volume, and 4) gas is continuously relaxed at rate G due to the combined effects of RF excitation, collisions with O2 molecules and uptake, and this rate is approximately constant during the breathing cycle. We then solve for the set of local Vn and FRC consistent with differential equations describing this model:$$$\frac{\dot{S}}{S}=\frac{\dot{V}}{V} \left(\frac{S_0}{S}-1\right)-\Gamma$$$ (inhale) and $$$\frac{\dot{S}}{S}=\frac{\dot{V}}{V} \left(\frac{V}{V-1+FRC}-1\right)-\Gamma$$$ (exhale) and fit to sigmoid curves describing local expansion, contraction rate and timing: $$$V_{n}=\frac{TV}{1+e^{-s_{I}\left(t_{n}-t_{I}\right)}}+FRC$$$ (inhale) and $$$ V_{n}=\frac{TV}{1+e^{s_{E}\left(t_{n}-t_{E}\right)}}+FRC$$$ (exhale). The resulting $$$S_{I}$$$ and $$$S_{E}$$$ describe the local rate of volumetric expansion, while $$$t_{I}$$$ and $$$t_{E}$$$ correspond to the times of peak flow into and out of the local region, respectively. Moreover, the first derivative of the volume flow over time gives the gas flow rate, allowing us to calculate the maximum flow rate = $$$\frac{-TV\cdot s}{4}$$$ .Results/Discussion
Figure 1 shows separate sigmoid fits for exhalation/inhalation. Three parameters were obtained for each breathing phase: maximum flow rate, time of maximum flow rate relative to the trachea, and the local rate of volumetric expansion, s. The color scale for the dynamic maps is not fixed since all these parameters depend on subjects’ different breathing patterns, but still shows local changes within subjects. While not observed in the healthy pig, Figure 2 shows a contrast between the right, tethered lung and the left lung in the TIS pig, where lower inhale s indicates less efficient gas replacement in the right lung, perhaps due to restriction associated with tethering. Interestingly, similar effects are seen in the ADC maps (white arrows), indicating that regions with high s might also have high diffusion, due to reduced microstructure size in the restricted lung and/or compensatory expansion in the contralateral lung. Figure 3 shows these maps for the human dataset, in which a superior-to-inferior gradient is noticeable for the flow rate parameters for all subjects except for the native left fibrotic lung of the unilateral LTX patient, analogous to the loss of ventilation gradients in severe emphysema. There was also a noticeable posterior-to-anterior gradient in the arrival/departure time in one of the bilateral LTX subjects (red arrows) consistent with expectations based on previous measurements of gravity-dependent sequential filling and emptying. This gradient was not seen in the other LTX subject. More work is required to understand these gradients and the correlation of s with ADC for the lung parenchyma.Conclusion
Dynamic HXe MR images can be analyzed to yield information about altered ventilation dynamics resulting from TIS and LTx, suggesting that these ventilation maps obtained during free breathing can assess the abnormal air flow and potentially allow for an improved assessment of altered lung dynamics associated with TIS and LTX.Acknowledgements
No acknowledgement found.References
1. Hamedani, H., et al. “Quantifying Fractional and out-of-Phase Ventilation Using Dynamic Hyperpolarized Xenon MRI during Free Breathing.” B28. SEE FOR YOURSELF: EVALUATING LUNG FUNCTION WITH CT AND MRI, 2022, https://doi.org/10.1164/ajrccm-conference.2022.205.1_meetingabstracts.a2541.Figures
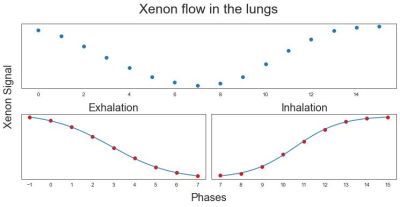

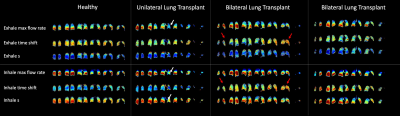
Figure 3. Ventilation maps for human LTx recipients, showing a vertical gradient in the maximum flow rates and s for all subjects, except in the fibrotic left lung of the unilateral lung transplant recipient (white arrows), analogous to the loss of ventilation gradients in severe emphysema. There was also a noticeable posterior-to-anterior gradient in the time shift in one of the bilateral LTX subjects (red arrows) consistent with expectations based on previous measurements of gravity-dependent sequential filling and emptying. This gradient was not seen in the other LTX subject.