4092
Interleaved multinuclear Na+-H+-CEST-EPI metabolic MRI for simultaneously quantifying salinity and acidity
Chencai Wang1,2, Xiaodong Zhong3, Alfredo L. Lopez Kolkovsky4, Sonoko Oshima1,2, Saneel Khairnar2,5, and Benjamin M Ellingson1,2,6,7
1Department of Radiological Sciences, UNIVERSITY OF CALIFORNIA, LOS ANGELES, Los Angeles, CA, United States, 2Brain Tumor Imaging Laboratory, University of California, Los Angeles, Los Angeles, CA, United States, 3MR R&D Collaborations, Siemens Medical Solutions USA, Los Angeles, CA, United States, 4Institute of Myology, Paris, France, 5Department of Molecular, Cell, and Developmental Biology, UNIVERSITY OF CALIFORNIA, LOS ANGELES, Los Angeles, CA, United States, 6Department of Neurosurgery, University of California, Los Angeles, Los Angeles, CA, United States, 7Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, United States
1Department of Radiological Sciences, UNIVERSITY OF CALIFORNIA, LOS ANGELES, Los Angeles, CA, United States, 2Brain Tumor Imaging Laboratory, University of California, Los Angeles, Los Angeles, CA, United States, 3MR R&D Collaborations, Siemens Medical Solutions USA, Los Angeles, CA, United States, 4Institute of Myology, Paris, France, 5Department of Molecular, Cell, and Developmental Biology, UNIVERSITY OF CALIFORNIA, LOS ANGELES, Los Angeles, CA, United States, 6Department of Neurosurgery, University of California, Los Angeles, Los Angeles, CA, United States, 7Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Keywords: Pulse Sequence Design, Non-Proton, Sodium, 2-Nuclei
Extensive evidence suggests abnormal metabolism, sodium homeostasis, and tumor acidity are interconnected and play critical roles in brain tumor formation, progression, seizure activity, treatment resistance, and immune suppression. While Na+ and advanced H+ -based MRI techniques can provide this information, Na+ and H+ images are traditionally acquired sequentially, resulting in a total scan time exceeding what is clinically reasonable. In the current study, we demonstrate utility of a new interleaved sodium gradient echo and proton-based pH-sensitive amine chemical exchange saturation transfer echoplanar imaging (Na+-GRE/H+-CEST-EPI) sequence in 15 minutes, making it feasible to study patients with brain tumors.Purpose
Extensive in vitro, preclinical, and clinical evidence have shown that abnormal metabolism, sodium homeostasis, and tumor acidity are interconnected and play critical roles in brain tumor formation, progression, seizure activity, treatment resistance, and immune suppression. While Na+ and advanced H+-based MRI can provide this information, Na+ and H+ images are traditionally acquired sequentially, resulting in a total scan time (approximately 20 to 25 minutes) exceeding what is clinically reasonable. In the current study, we developed a new interleaved sodium gradient echo and proton-based pH-sensitive amine chemical exchange saturation transfer echoplanar imaging (Na+-GRE/H+-CEST-EPI) sequence in approximately 15 minutes, making it clinically and economically feasible, particularly to study patients with brain tumors.Methods
Phantom Preparation: The phantom was composed 3 glycine phantom solutions (pH~6.00 with different [Na+] of ~1.00M, ~0.50M and ~0.25M) and 2 deionized water (pH=7.00 with different [Na+] of ~1.00M and ~0.50M), all stored in Nalgene 30 ml polypropylene bottles (Thermo Fisher Scientific, Waltham, MA, USA). The detailed composition, including Na+ concentration and measured pH of each sample were summarized in Table 1.Table 1 Phantom solution composition
| Sample | Volume | Phosphate | Glycine | K2HPO4/KH2PO4 Ratio | Measured pH | Na+ |
| #1 | 50.00 mL | 10.00 mM | 20.00 mM | 0.19 | 5.99 | 1.00 M |
| #2 | 50.00 mL | 10.00 mM | 20.00 mM | 0.19 | 6.01 | 0.50 M |
| #3 | 50.00 mL | 10.00 mM | 20.00 mM | 0.19 | 6.00 | 0.25 M |
| #4 | 50.00 mL | 10.00 mM | 20.00 mM | 0.19 | 7.00 | 1.00 M |
| #5 | 50.00 mL | 10.00 mM | 20.00 mM | 0.19 | 7.00 | 0.50 M |
MRI Acquisition: MRI measurements were performed on a Siemens 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a custom multinuclear coil (1-channel Na+/1-channel H+, RAPID, Columbus OH). Na+ and pH-sensitive images were acquired by a custom sequence performing interleaved acquisitions of a Na+-GRE and H+-CEST-EPI (Figure 1). Acquisition parameters included field-of-view (FOV)=500×500mm, matrix size=128×128, slice thickness=2.8mm with 50% interslice gap, bandwidth=902Hz/Pixel, partial Fourier=6/8, TE/TR=4.0ms/6.6ms for Na+-GRE, and TE/TR=48ms/500ms for H+-CEST-EPI. CEST off-resonance saturation was applied using a pulse train of 3 × 100 ms Gaussian pulses with peak amplitude of 6µT [1]. A total of 29 CEST off-resonance frequencies were sampled at −3.5 to −2.5ppm, −0.3 to +0.3 ppm, and +2.5 to +3.5ppm, with increments of 0.1 ppm. Four reference S0 scans were collected with the same acquisition parameters, without the saturation pulses. Additionally, high-resolution T1-weighted MPRAGE images were acquired for anatomic reference.
CEST Image Processing: Following the B0 correction via a z-spectra based k-means clustering and Lorentzian fitting algorithm [2], an integral width of 0.4 ppm was taken around both the −3.0 and +3.0 ppm spectral points of the inhomogeneity-corrected data. These data points were combined with the S0 image to calculate the asymmetry in the magnetization transfer ratio (MTRasym) at 3.0 ppm as defined by equation MTRasym(ω) = S(−ω)/S0 − S(ω)/S0, where ω is the offset frequency of interest (3.0 ppm). The signal-to-noise ratio (SNR) of each phantom sample was calculated using the division of the mean signal intensity over the standard deviation of signal intensity.
Results
The Na+-GRE sequence was interleaved with the H+-CEST-EPI sequence for each image slice (Figure 1). Figure 2 shows phantom results, demonstrating the ability to construct Na+ (33 averages) and pH-weighted CEST-EPI (MTRasym at 3.0ppm) images simultaneously. The scan time for one slice of Na+ (33 averages) and pH-weighted CEST-EPI (MTRasym at 3.0ppm) images is 37 seconds. Therefore, the total scan time for interleaving Na+ GRE and H+-CEST-EPI of 25 image slices is approximately 15 minutes. In contrast, besides shimming time, the total scan time for H+-CEST-EPI of 25 image slices only was approximately 7.5 minutes, and the total scan time for Na+-GRE of 25 image slices only was approximately 12 to 19 minutes. Using the interleaved Na+-GRE/H+-CEST-EPI sequence, the SNR of the CEST image for five phantom samples were 7.38, 6.34, 8.88, 8.82, and 7.84, respectively (7.87±1.05). In contrast, using the clinical H+-CEST-EPI sequence [1], the SNR of the CEST image for five phantom samples were 6.58, 7.86, 7.22, 11.26, and 4.34, respectively (7.46±2.51).Discussion
Results demonstrate that an interleaved Na+-GRE/H+-CEST-EPI sequence allows for simultaneous acquisition of sodium- and pH-weighted molecular MR images. These images can be acquired in clinically reasonable scan times and with no added contrast or risk to patients. This innovative approach will potentially be useful for understanding brain tumor biology, including identifying unique and exploitable metabolic characteristics and evaluating new drugs that may impact or be influenced by tumor metabolism.Acknowledgements
This project was funded by DOD CA200290 and the UCLA Sodium MRI Program. We also thank Dr. Nathanson's lab to provide the workspace for preparing phantom.References
1. Harris RJ, Cloughesy TF, Liau LM, Nghiemphu PL, Lai A, Pope WB, et al. Simulation, phantom validation, and clinical evaluation of fast pH-weighted molecular imaging using amine chemical exchange saturation transfer echo planar imaging (CEST-EPI) in glioma at 3 T. NMR Biomed. 2016;29:1563-76.2.
2. Yao J, Ruan D, Raymond C, Liau LM, Salamon N, Pope WB, et al. Improving B0 Correction for pH-Weighted Amine Proton Chemical Exchange Saturation Transfer (CEST) Imaging by Use of k-Means Clustering and Lorentzian Estimation. Tomography. 2018;4:123-37.
Figures
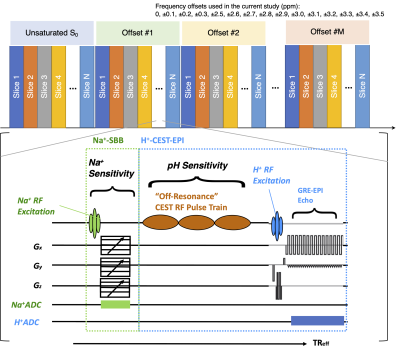
Figure 1. Sequence diagram for the interleaving multinuclear Na+-GRE/H+-CEST-EPI sequence.
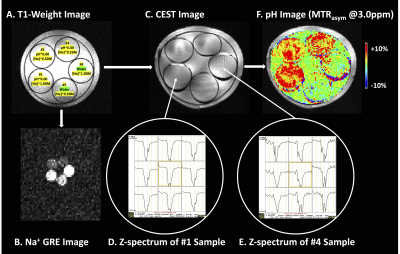
Figure 2. A) T1-weighted image with the pH and Na+ concentration labeled for each phantom sample. B) Na+ GRE (average over 33 scans) and C) CEST images can be constructed simultaneously. Using D) and E) voxel-wise Z-spectral data of different phantom samples, F) pH image (MTRasym @3.0ppm) can be calculated.
DOI: https://doi.org/10.58530/2023/4092