4076
Preliminary SNR comparison between the 11.7T Iseult RF coil and its twin coil at 7T1NeuroSpin, CEA, Gif-sur-Yvette, France, 2Paris-Saclay University, Gif-sur-Yvette, France, 3Baobab, CNRS, Gif-sur-Yvette, France, 4IRFU, CEA, Gif-sur-Yvette, France
Synopsis
Keywords: High-Field MRI, High-Field MRI, 11.7T, Signal-to-Noise Ratio
The Iseult coil, developed for MRI of the human brain at 11.7T, was presented at last year’s ISMRM meeting. Here we show SNR phantom measurements in comparison with the Avanti2 coil, a replica of the Iseult coil tuned at 300 MHz (for 7T). We derive a new power dependence of the SNR vs B0.Introduction
In the framework of the Iseult 11.7T project, a parallel transmit RF coil was designed for the human brain1 with a minimized outer diameter (27 cm) to fit in a local B0 shim device, the so-called SCOTCH multi-coil array2. This Iseult head coil combines 15 transceive and 17 receive-only elements, all geometrically decoupled via Resonant Inductive Decoupling elements3 (no preamp-decoupling). Yet it is currently used with a transmit array limited to 8 power channels. Here we want to quantify its SNR and compare it with that obtained at 7T from a twin coil called Avanti2. Avanti2 and Iseult share the same size and hybrid architecture with 2 rows of alternate loops and small dipoles (in fact air-gap center-fed microstrips), and a patch at the top of the head. Their receive elements are all matched to 50Ω, and tuned to 298.2 and 499.4 MHz, respectively. The preamplifiers of these elements also share similar noise figures (measured NF ~ 0.6 for Avanti2, ~0.5 for Iseult). Therefore, comparison of the mean SNR of these coils in any sample at 11.7T and 7T can be used to infer the B0-strength power dependence of the SNR. This is what we attempt to do here with a salted Agar phantom, as in-vivo scanning is not authorized yet at 11.7T.Methods
A doped 16-cm Agar sphere was used for SNR measurement. Its electric properties were measured to be εr ~ 70 and σ ~ 0.9 S/m at both frequencies of interest. From a single voxel spectroscopy sequence repeated with several TRs, its T1 was found to be {1420, 1470} ms at {7, 11.7} T respectively. Both the 7T and 11.7T scanners at NeuroSpin are equipped with the Siemens SC72 whole-body gradient system. The protocol for SNR measurement was the same at 7T and 11.7T, and followed these steps :- After B0-shimming, acquire B0 and multi-channel B1 maps from 3D triple-echo Gradient Echo (GRE) and interferometric pre-saturated 2D turbo-FLASH (XFL)4 sequences, respectively. The B0 map has a 2.5-mm isotropic resolution, whereas B1 maps are acquired with 5-mm voxels.
- To get a homogeneous excitation throughout the whole phantom, design a 30°-flip angle pTx pulse based on 6 kT-points5 with maximum 2-ms total duration, based on the collected B0 and B1 maps.
- Run a 3D GRE sequence loaded with the kT-points pulse. Sequence parameters are: transverse acquisition, TE=2.5ms, TR = 75ms, 2.5-mm isotropic resolution, matrix size: 128x96x96, readout bandwidth 64 kHz.
- Collect RF noise from this sequence by setting pulse voltage to zero, and derive the noise covariance matrix.
- From the k-space data and noise covariance matrix, pre-whiten the data before sum-of-square combination.
- Correct the obtained SNR map for varying Flip Angles (FA) by using the FA-maps obtained from a Bloch simulator and the measured field maps (B0 and B1 from individual Tx-channels). Use the T1 values above. Thus find the corrected 90°-FA infinite-TR SNR.
- Apply thresholds to the uncorrected SNR map for determination of the voxels in the phantom (3D masking).
- Realignment and intersection of the derived ROIs lead to common masks from which mean corrected SNRs are compared.
Results
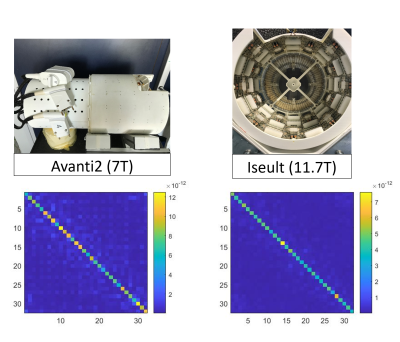
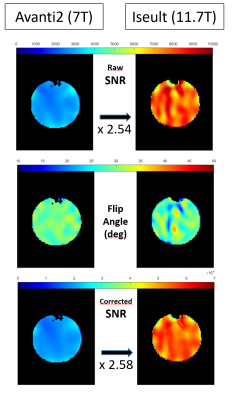
Fig. 1 shows the magnitude of the Noise Covariance matrices for each coil. The clean off-diagonal coefficients, especially in the Iseult RF coil, are caused by 50-W power-matching between Rx elements and their preamplifier6.Fig. 2 depicts the SNR in the central axial slice before and after FA-variation correction for the two RF coils under investigation. Also shown are the FA maps calculated from the kT-points Bloch simulation. Note the larger dispersion of the simulated FA around 30° across the phantom for Iseult: the calculated normalized RMS FA errors are 8.3% and 12.7% at 7T and 11.7T, respectively. Now consider the 2.58 factor between Avanti2 and Iseult mean SNR after FA correction. Recalling both coils have identical features, a 1.83-power law between SNR and field strength can be derived from this result.
Discussion
Our SNR results are somewhat consistent with Le Ster et al7, considering the entire 16-cm spherical phantom is used here for SNR-averaging. This probably explains the slightly lower field power dependence of the SNR obtained here (1.83 vs 1.94 in 7), since only the central voxels were considered in that reference when exciting with a volume coil: this is where the dielectric resonance effect caused by the Circularly-Polarized mode enhances the signal the most with growing field strength. Future work involves correcting for the flip angle excitation by using an AFI sequence8, instead of a Bloch simulation. Its implementation was attempted in this work but returned unreliable results due to what is believed to be a field oscillation induced by a strong vibration of the gradient, itself caused by strong spoilers juxtaposed to the phase sensitive pTx pulses.Conclusion
When comparing Rx performance of the Iseult RF head coil to its 7T counterpart in an aqueous gel, a preliminary 2.58 factor increase of the mean SNR is observed, hinting at a 1.83 field power coefficient for Agar phantoms. For human brains, we expect a slightly lower power value because of a lower relative permittivity (43 vs 70)9. This will be the topic of a future study when in-vivo imaging authorizations are granted at 11.7T.Acknowledgements
No acknowledgement found.References
1. Luong M, et al, A Compact 16Tx-32Rx Geometrically Decoupled Phased Array for 11.7T MRI, ISMRM 2022, #707.
2. Pinho Meneses B, et al, Shim coils tailored for correcting B0 inhomogeneity in the human brain (SCOTCH): Design methodology and 48-channel prototype assessment in 7-Tesla MRI, NeuroImage 261:119498, 2022.
3. Avdievich N, et al. Resonant Inductive Decoupling (RID) for Transceiver Arrays to Compensate for both Reactive and Resistive Components of the Mutual Impedance. NMR Biomed; 26(11):1547-54, 2013.
4. Amadon A, et al, Validation of a very fast B1-mapping sequence for parallel transmission on a human brain at 7T, ISMRM 2012, #3358.
5. Cloos M A, et al. KT-points: Short Three-Dimensional Tailored RF Pulses for Flip-Angle Homogenization over an Extended Volume, Magn Reson Med, 67(1):72-80, 2012.
6. Gapais, P-F, et al, On the Noise Correlation in Receive Phased Arrays, ISMRM 2022, #1537.
7. Le Ster, C, et al, Magnetic field strength dependent SNR gain at the center of a spherical phantom and up to 11.7T, Magn Reson Med, 88(5):2131–2138, 2022.
8. Yarnykh V, Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field, Magn Reson Med 57(1):192-200, 2007.
9. Pohmann R, et al, Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays, Magn Reson Med, 75(2):801-9.
Figures