4060
Imaging of the Contraction Pattern of Spontaneous Muscular Activities using a Multiple-Point Diffusion-Weighted Stimulated Echo Sequence1Section on Experimental Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany
Synopsis
Keywords: Muscle, Muscle
Spontaneous mechanical activities in resting musculature can be visualized using stimulated-echo or spin-echo diffusion-weighted sequences. Temporal resolution is limited to the sampling of one point in time of the contraction pattern. Therefore, a concept for multiple points in time imaging of spontaneous activities is proposed in this work. The implemented MR sequence was validated by measurements from a water phantom and of the resting lower leg musculature of healthy subjects.Introduction
Diffusion-weighted stimulated-echo (DW-STE) imaging is able to visualize spontaneous mechanical activities in musculature (SMAM)1 as signal voids. Common approaches applying a standard DW-STE1-4 or spin-echo5 sequence are only able to sample the muscular contraction at one point in time. Thus, visualization of the course of contraction over time is not provided. On the other hand, gaining further knowledge on the time course of muscle twitches is desired, e.g., contraction and relaxation times. Standard DW-STE imaging can be extended with a series of low flip angle excitations for high-speed imaging6,7 or multi-slice imaging8; however, imaging of the time course of muscular contraction is still impossible.Therefore, a STE technique for multiple points in time diffusion-weighted (MP-DW) imaging of a single muscular contraction is introduced. Preliminary results for validation of the novel MP-DW-STE sequence are given from phantom measurements and MR examination of healthy subjects.
Design of the DW-MR Sequence
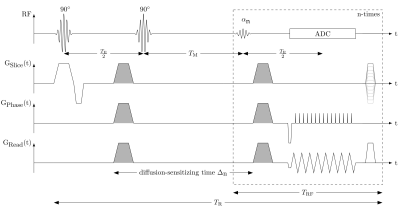
The sequence scheme of the single-shot MP-DW-STE imaging sequence is depicted in Fig. 1. In contrast to the conventional STE scheme, the third RF pulse after the mixing time $$$T_M$$$ which deflects the stored magnetization into the transverse plane is replaced by a series of $$$N_{RF}$$$ variable flip angles $$$\alpha_n=[\alpha_1, ..., \alpha_{N_{RF}}], 0<\alpha<90°\forall n\in \{1, ..., N_{RF}\}$$$. Thus, each pulse deflects only a fraction of the stored magnetization. Before the signal echo is sampled, the rephasing gradient lobe for diffusion weighting is inserted. In contrast to previous works8, the whole k-space is read out by an echo-planar imaging (EPI) trajectory achieving the acquisition of $$$N_{RF}$$$ single-shot images. To avoid destructive interference of the transverse magnetization on signal echoes in subsequent imaging cycles, a spoiler gradient pulse along the slice selection axis $$$G_{Slice}$$$ with random amplitude and a dephasing gradient pulse along the readout axis $$$G_{Read}$$$ are inserted in each imaging cycle at the end of $$$T_{RF}$$$. Since an increase of the echo train length leads to enhanced sensitivity to imaging artifacts, the EPI readout was shortened by applying ramp sampling with a constant dwell time9. The signal intensity of the $$$n$$$-th acquisition is$$M_n = \frac{M_0}{2}e^{-\frac{T_M+(n-1)T_{RF}}{T_1}}\prod_{k=1}^{n-1}cos(\alpha_k)e^{-\frac{T_E}{T_2}}\cdot sin(\alpha_n).$$During the mixing interval $$$T_M$$$, the stored magnetization in longitudinal direction relaxes depending on the $$$T_1$$$ value of the tissue. Cyclic readout of the stored magnetization with $$$T_{RF}$$$ leads to an increase of $$$T_M: T_{M,n}=T_M+(n-1)T_{RF}$$$. Thus, the signal intensity in each voxel changes over the imaging cycles. This effect can be compensated by a variable flip angle $$$\alpha_n$$$ deflecting the same amount of magnetization in each cycle. This leads under ideal conditions to a constant signal intensity for a given $$$T_1$$$. Given the condition that the entire remaining stored magnetization should be deflected in the last cycle $$$N_{RF}$$$ with $$$\alpha_{N_{RF}}=90°$$$, the flip angle series $$$\alpha_n$$$ is iteratively determined following $$\alpha_n = arctan\bigg(e^{-\frac{T_{RF}}{T_1}}sin(\alpha_{n+1})\bigg).$$ Increasing $$$T_{M,n}$$$ leads to an increase of the diffusion-sensitizing time, and thus to a change of the diffusion-sensitivity of each imaging cycle. The b-value of the $$$n$$$-th image acquisition is $$b_n=\gamma^2G_D^2\bigg[\delta^2(\Delta_n-\frac{\delta}{3})+\frac{\xi^3}{30}-\frac{\delta\xi^2}{6}\bigg]$$ with $$$\Delta_n = \Delta_0+(n-1)T_{RF}$$$ ($$$\Delta_0$$$: initial diffusion-sensitizing time of the first acquisition; $$$\xi$$$: rise time; $$$\delta$$$: length of diffusion gradient). The described MR pulse sequence was implemented with Pulseq10 on a 3T MR system (MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany). All image reconstruction steps are implemented in custom-written code in MATLAB® (The MathWorks, Natick, MA, USA).Evaluation of DW-MR Sequence
The implemented MP-DW-STE sequence was evaluated using a standard water phantom and in-vivo measurements conducted on five healthy subjects (age: 40±16 years) with a flip angle series $$$\underline{\alpha}=[23.7°, 27.5°, 33.2°, 43.6°, 90°]$$$ ($$$T_1=1200$$$ ms). Spontaneous muscular activities were analyzed using a neural network-based approach11.Results & Discussion
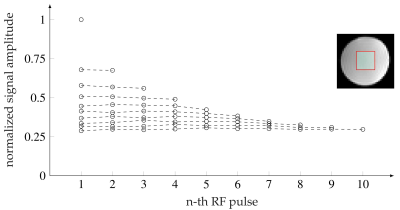
In Fig. 2, the flip angle series for 1 to 10 repetitions is given with the normalized signal intensity inside a water phantom (Fig. 3). The highest variation of the signal amplitude within a RF series was ±0.0127 (mean normalized signal: 0.445) for $$$N_{RF}=5$$$ and lowest for $$$N_{RF}=10$$$ with ±0.005 (mean normalized signal: 0.298).Fig. 4A shows an exemplary time-series of DWI with distinct SMAM in the m. soleus (SOL). The active area of the SMAM reaches its maximum cross-sectional area (CSA) at the 3rd imaging point in time (III). In relation to this maximum extension, the area of the SMAM in the image time-series was: 72.7% (I), 92.4% (II), 100% (III), 84.8% (IV) and 50.0% (V). Six MP-DWI repetitions with more than four SMAM-affected points in time in the SOL from one subject were utilized for estimating muscular contraction parameters (Fig. 4B). A contraction model4,12 was fitted to the course of CSA. A contraction time $$$T_C=119.9$$$ ms was found for the given data points. In 14% of all DWI time-series we found activities ($$$N_{SMAM}=60$$$) where 36.7% ($$$N_{SMAM}=22$$$) affected all five images (four: 31.2%/19, three: 8.3%/5, two: 8.3%/5, one: 15.0%/9). Images with only one SMAM-affected DWI were mostly acquired at the last point in time (66.7%).
Conclusion
Preliminary in-vivo results on healthy subjects demonstrate that the acquisition of several points in time of the human muscular twitch contraction is feasible. Parts of unique muscle contractions could be visualized in a single-shot acquisition scheme. For a more accurate characterization, fast sampling of k-space is required to shorten the interval between two RF excitations allowing more sampling points within the acquisition window.Acknowledgements
This work was supported and funded by the German Research Foundation (DFG) under Grants SCHI 498/11‐2 and YA 28/16‐2.References
[1]: G. Steidle and F. Schick. Addressing spontaneous signal voids in repetitive single-shot DWI of musculature: spatial and temporal patterns in the calves of healthy volunteers and consideration of unintended muscle activities as underlying mechanism. NMR in Biomedicine, 28:801–810, July 2015. ISSN 1099-1492. doi: 10.1002/nbm.3311.
[2]: M. Schwartz, G. Steidle, P. Martirosian, A. Ramos-Murguialday, H. Preissl, A. Stemmer, B. Yang, and F. Schick. Spontaneous mechanical and electrical activities of human calf musculature at rest assessed by repetitive single-shot diffusion-weighted MRI and simultaneous surface electromyography. Magnetic Resonance in Medicine, 9:2784–2794, May 2018. ISSN 1522-2594. doi: 10.1002/mrm.26921.
[3]: M. Schwartz, P. Martirosian, G. Steidle, M. Erb, A. Stemmer, B. Yang, and F. Schick. Volumetric assessment of spontaneous mechanical activities by simultaneous multi-slice MRI techniques with correlation to muscle fiber orientation. NMR in Biomedicine, 31:e3959, Nov. 2018. ISSN 1099-1492. doi: 10.1002/nbm.3959.
[4]: M. Schwartz, P. Martirosian, G. Steidle, T. Feiweier, B. Yang, and F. Schick. Capability of Diffusion-Weighted Stimulated-Echo Imaging to Visualize Spontaneous Muscular Activities in Resting Musculature. In Proceedings of the Annual Meeting International Society for Magnetic Resonance in Medicine (ISMRM), 2022
[5]: M. G. Birkbeck, L. Heskamp, I. S. Schofield, A. M. Blamire, and R. G. Whittaker. Non-invasive imaging of single human motor units. Clinical Neurophysiology, 131:1399–1406, June 2020. ISSN 1872-8952. doi: 10.1016/j.clinph.2020.02.004.
[6]: K. D. Merboldt, W. Hänicke, and J. Frahm. Diffusion imaging using stimulated echoes. Magnetic Resonance in Medicine, 19:233–239, June 1991. ISSN 0740-3194. doi: 10.1002/mrm.1910190208.
[7]: K. D. Merboldt, W. Hänicke, H. Bruhn, M. L. Gyngell, and J. Frahm. Diffusion imaging of the human brain in vivo using high-speed STEAM MRI. Magnetic Resonance in Medicine, 23:179–192, Jan. 1992. ISSN 0740-3194. doi: 10.1002/mrm.1910230119.
[8]: J. Frahm, K.-D. Merboldt, W. Hänicke, and A. Haase. Stimulated Echo Imaging. Journal of Magnetic Resonance, 64:81–93, 1985.
[9]: D. Q. Chen, R. B. Marr, and P. C. Lauterbur. Reconstruction from NMR data acquired with imaging gradients having arbitrary time dependence. IEEE Transactions on Medical Imaging, 5:162–164, 1986. ISSN 0278-0062. doi: 10.1109/TMI.1986.4307765.
[10]: K. J. Layton, S. Kroboth, F. Jia, S. Littin, H. Yu, J. Leupold, J.-F. Nielsen, T. Stöcker, and M. Zaitsev. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magnetic Resonance in Medicine, 77:1544–1552, Apr. 2017. ISSN 1522-2594. doi: 10.1002/mrm.26235.
[11]: M. Schwartz, T. Küstner, P. Martirosian, J. Machann, G. Steidle, B. Yang, and F. Schick. Robust Quantification of Spontaneous Muscular Activities by Simultaneous Interpretation of sEMG Data. In Proceedings of the 36th Annual Scientific Meeting European Society for Magnetic Resonance in Medicine and Biology (ESMRMB), 2019.
[12]: R. D. Herbert and S. C. Gandevia. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. Journal of Neurophysiology, 82:2271–2283, Nov. 1999. ISSN 0022-3077. doi: 10.1152/jn.1999.82.5.2271.
Figures
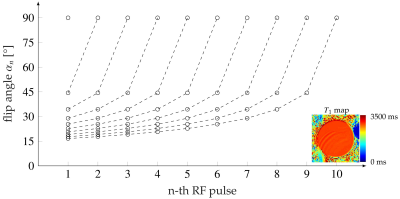
Flip angle $$$\alpha_n$$$ for the image acquisition of the $$$n$$$-th repetition ($$$n=1, ..., 10$$$) using $$$T_1=2800$$$ ms (determined from bSSFP mapping). At the bottom right, the $$$T_1$$$ map determined from the bSSFP sequence is shown.
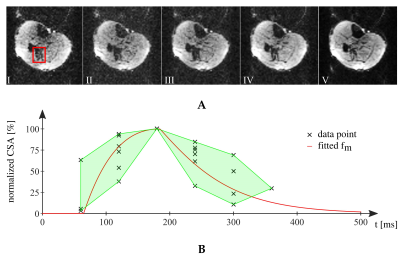
A: Exemplary MP-DW-STE image series showing the time course of a distinct spontaneous muscle activity (highlighted in red) in SOL from one subject. The SMAM grows from 72.7% (1st image) over 100% (3rd ) and relaxes to 50% (5th). B: Normalized CSA of data points from SOL with fitted course of muscular contraction following Herbert et al.12 and highlighted min-max region (optimized parameter: $$$T_C=119.9$$$ ms).