4056
Relationship between the properties of paraspinal muscles and bone mineral density in patients with lumbar disc herniation1Chengdu Sport University, Chengdu, China, 2GE Healthcare China, Beijing, China, 3Sichuan Province Orthopedic Hospital, Chengdu, China
Synopsis
Keywords: Muscle, Fat, Quantitative imaging, Paraspinal muscle
Paraspinal muscles and vertebral bodies are important to maintain spinal stability. Muscles and bones are functional tissues that interact with each other. We investigated the relationship between the properties of paraspinal muscles obtained from MR imaging and bone mineral density of vertebral bodies measured from quantitative CT in patients with lumbar disc herniation (LDH). Our results showed that the fat fraction of paraspinal muscles was negatively correlated with bone mineral density of vertebral bodies in LDH patients, and the fat fraction of multifidus muscle at the L3/L4 lumbar disc level may be the optimal predictor of bone mineral density changes.Introduction
Osteoporosis is a systemic metabolic bone disease closely related to age, which is characterized by decreased bone mass and changes in bone microarchitecture (1). Osteoporosis increases the bone fragility and the risk of vertebral fractures. Paraspinal muscles play an important role in maintaining spinal stability. The weakening of lumbar muscle strength is also a factor that leads to the occurrence of vertebral fractures. The muscle cross-sectional area (CSA) and fat infiltration are two common imaging indicators to evaluate paraspinal muscle strength. Muscles and bones are functional tissues that are interconnected and interact with each other (2). A recent study demonstrated that the properties of paraspinal muscles are related to volumetric bone mineral density (vBMD) in patients with degenerative lumbar spine (3), where the fat infiltration of paraspinal muscles was indirectly evaluated on routine T2-weighted images. In contrast, chemical shift-encoded IDEAL-IQ (4) sequence can directly provide accurate fat fraction (FF) values of paraspinal muscles (5). In this study, we investigated the relationship between the properties of paraspinal muscles obtained from IDEAL-IQ sequence and volumetric bone mineral density in patients with lumbar disc herniation (LDH), and looked for the optimal predictor of bone mineral density change in LDH patients.Methods
Patients: After IRB-approved written informed consent was obtained, patients with low back pain were scanned on 3.0 T MRI (SIGNA Architect, GE Healthcare, USA) and CT (Siemens Somatom Definition AS+, Germany) to measure the properties of paraspinal muscles and vBMD, respectively. Finally, 121 subjects (aged 25-69 years, 64 females) with clinically and radiologically diagnosed LDH were enrolled in this retrospective study.MR imaging parameters: The MR scan included axial T2 FSE (0.5×0.8×3.0 mm3, TR=3224 ms, TE=120 ms), axial IDEAL-IQ (2.0×2.0×2.0 mm3, TR=8.0 ms, TE=3.6 ms, number of TEs=6, number of shots=2), for measuring the cross-sectional area (CSA) and fat fraction (FF) of paraspinal muscles, respectively.
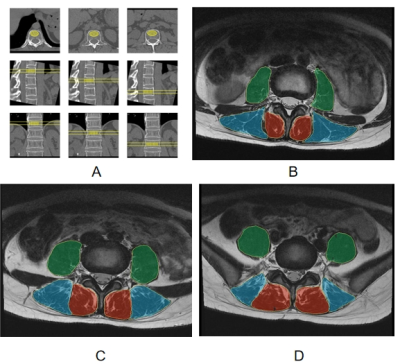
Data processing: CT Data were transmitted to the QCT Pro workstation to measure the mean vBMD of three vertebral bodies at T12, L1, and L2 levels (Figure 1A). The IDEAL-IQ images were processed in the AW4.7 workstation (GE Healthcare) to calculate the FF map. The mean CSA and FF values of the bilateral paraspinal muscles were obtained on a ROI basis at the central level of L3/L4, L4/L5, and L5/S1 (Figure 1B-1D), respectively.
Statistical analysis: All analyses were performed using SPSS 22.0 software. Patients were divided into normal bone mass (vBMD>120 mg/cm3) and abnormal bone mass (vBMD<120 mg/cm3) groups according to the International Society for Clinical Densitometry in 2007 (6). The differences of BMI, age, paraspinal muscle CSA and FF values between the two groups were tested by independent samples T-test. Fisher's exact test was used to compare the gender composition. The correlations between the properties (CSA and FF) of paraspinal muscles and vBMD were analyzed using Pearson correlation coefficients.
Results and discussion
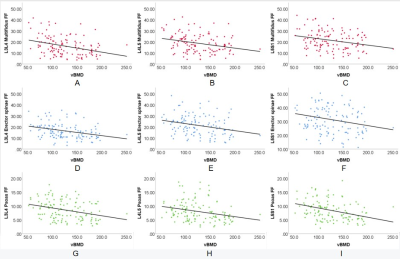
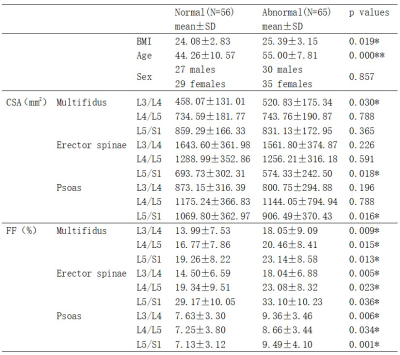
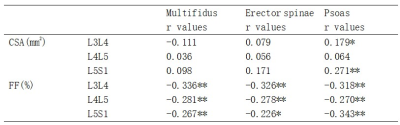
As shown in Table 1, among the 121 LDH patients, 56 had normal bone mass and 65 had abnormal bone mass. No significant difference was found in the CSA of the paraspinal muscles between LDH patients with normal and abnormal bone mass, except for multifidus at L3/L4, erector spinae at L5/S1, and psoas at L5/S1 (p<0.05). The FF values of all three paraspinal muscles at all three levels in patients with abnormal bone mass were significantly higher than those in patients with normal bone mass (p<0.05), which may be explained by the higher BMI and age of the former patients.As shown in Table 2, no correlation was found between the CSA of paraspinal muscles and vBMD, except for psoas at L3/L4 (p<0.05) and psoas at L5/S1 (p<0.001). The FF was negatively correlated with vBMD in all three paraspinal muscles at all three levels (p<0.001). That is, the degree of FF in paraspinal muscles increased with the decrease of vBMD in vertebral bodies (Figure 2), suggesting that the degree of paraspinal muscle fat infiltration may be a marker of low bone mineral density. Interestingly, multifidus muscle at the L3/L4 level had the highest correlation coefficient (Table 2), indicating that the fat fraction of multifidus muscle at the L3/L4 level may be the optimal predictor of bone mineral density changes in LDH patients.
In future clinical work, changes in lumbar volumetric bone mineral density may be predicted by adding the IDAEL-IQ sequence during MR scan, without the use of CT scan with ionizing radiation. Further prospective studies are needed to investigate the possibility of reducing bone loss by reducing fat infiltration in the paraspinal muscles through intervention, such as functional training of paraspinal muscles.
Conclusion
The fat fraction of lumbar paraspinal muscles was negatively correlated with the volumetric bone mineral density of vertebral bodies in LDH patients, and the degree of fat infiltration in paraspinal muscles at the L3/L4 level may be the optimal predictor of bone mineral density changes.Acknowledgements
No acknowledgement found.References
1. Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014;25:1439–1443.
2. Tagliaferri C, Wittrant Y, Davicco M-J, et al. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015;21:55–70.
3. Han G, Zou D, Liu Z, et al. Paraspinal muscle characteristics on MRI in degenerative lumbar spine with normal bone density, osteopenia and osteoporosis: a case-control study. BMC Musculoskelet. Disord. 2022;23:1–7.
4. Yu H, Shimakawa A, McKenzie CA, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2008;60:1122–1134.
5. Fischer MA, Nanz D, Shimakawa A, et al. Quantification of muscle fat in patients with low back pain: comparison of multi-echo MR imaging with single-voxel MR spectroscopy. Radiology 2013;266:555–563.
6. Baim S, Binkley N, Bilezikian JP, et al. Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J. Clin. Densitom. 2008;11:75–91.
Figures