4052
Investigating muscle micro-trauma with time-dependent diffusion and the random permeable barrier model1Department of Biomedical Engineering and Physics, Amsterdam Movement Sciences, Amsterdam UMC, location AMC, Amsterdam, Netherlands, 2C.J. Gorter MRI Center, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 3Department of Radiology and Nuclear Medicine, Amsterdam UMC, location AMC, Amsterdam, Netherlands, 4Department of Radiology, Utrecht UMC, Utrecht, Netherlands
Synopsis
Keywords: Muscle, Diffusion Tensor Imaging
Repetitive muscle micro-trauma can result in severe muscle injuries. Diffusion tensor imaging can detect small but significant changes due to muscle micro-trauma, but the sensitivity is limited. Longer and multiple diffusion times can potentially increase the sensitivity to micro-trauma as they facilitate muscle fiber diameter and permeability estimations with the random permeable barrier model (RPBM). In this study, we demonstrated that a diffusion time of 116.7ms showed largest percentage change in DTI indices suggesting an increased sensitivity to exercise-induced muscle micro-trauma after running a marathon. No effect was found in the RPBM-derived membrane permeability and fiber diameter.Introduction
Repetitive muscle micro-trauma can result in severe muscle injuries, however, micro-trauma is hard to detect with conventional imaging techniques1. Diffusion tensor imaging (DTI) has been shown to be sensitive to micro-trauma2; however, the observed changes are small which makes detection in individual patients challenging.Conventional SE-DTI uses diffusion times in the order of 30-50ms, but given the typical muscle fiber diameter (30-80μm)3, choosing a diffusion time that probes at a larger length scale (>100ms) might increase the sensitivity to micro-trauma. Moreover, by adding multiple diffusion times estimations of muscle fiber diameter and membrane permeability can be made non-invasively via the random permeable barrier model (RPBM)4,5, which could also aid in micro-trauma detection.
In this study, we used DTI with multiple diffusion times to assess exercise-induced muscle micro-trauma. The aim of the study is twofold: 1. Investigate the differences in sensitivity of the DTI parameters to muscle micro-trauma at different diffusion times. 2. Explore the RPBM-derived parameters in muscle micro-trauma.
Methods
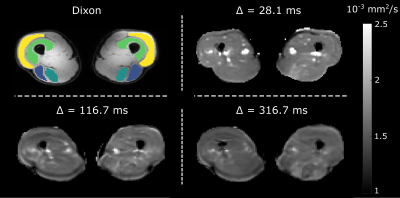
Nine male marathon runners (mean age 53.2±6.3 years) underwent MRI examinations (3T Philips Ingenia, Philips, Best, The Netherlands) of the upper leg muscles (mid-thigh level) at three time-points: one week before (TP-0), 24-48 hours after (TP-1) and two weeks after (TP-2) completing the Amsterdam Marathon.The scan protocol included an anatomical 4-point chemical-shift-based-water-fat-separation gradient echo (Dixon) scan (1.5x1.5x6mm³, 31 slices, SENSE-factor=2, TR=210ms, TE=2.6/3.36/4.12/4.88ms), a SE-EPI DTI scan (3x3x6mm³ resolution, 31 slices, TE/TR=57ms/6200ms, SENSE-factor 1.9, partial Fourier factor=0.73, b-values (directions): 0 (6), 200 (6), 400 (8), 600 (12) s/mm²) and two stimulated echo (STE) EPI DTI scans with mixing times of 100ms and 300ms (3.75x3.75x12mm³, 15 slices, TE/TR=34ms/8400ms, SENSE-factor=1.7, partial Fourier factor=0.7, b-values (directions): 0 (4) and 400 (24) s/mm²). The diffusion times (Δ) were 28.1ms for the SE-DTI and 116.7ms and 316.7ms for the STE-DTI acquisitions.
The data from all DTI scans were denoised via MP-PCA denoising6 and registered to the anatomical Dixon images using elastix7. A nonlinear-least-squares DTI fit was performed in Matlab (R2021a, The MathWorks, Natick, CA). For each voxel, the outcome parameters mean diffusivity (MD), fractional anisotropy (FA) and the tensor eigenvalues λ1, λ2 and λ3 were calculated.
ROIs were drawn manually in two hamstring and two quadriceps muscles in both legs: biceps femoris long head (BFL), semitendinosus (ST), vastus intermedius (VI) and vastus lateralis (VL) (Figure 1). Median DTI parameters were obtained per ROI for all time-points and diffusion times. Percentage change in DTI parameters between the time-points was calculated per muscle for all diffusion times to assess sensitivity to micro-trauma.
The RPBM model was fitted to the mean radial diffusivity (RD=(λ2+λ3)/2) per muscle. The axial diffusivity (AD=λ1) was fixed to the mean value of the STE-DTI data. This yielded the mean fiber diameter a and membrane permeability κ. For a the correction factor proposed by Berry et al8 was used. A Friedman test followed by a post-hoc Dunn’s test was used to assess differences in percentage change for the diffusion indices between the diffusion times and differences in RPBM-indices between muscles and time-points (significant at p≤0.05).
Results
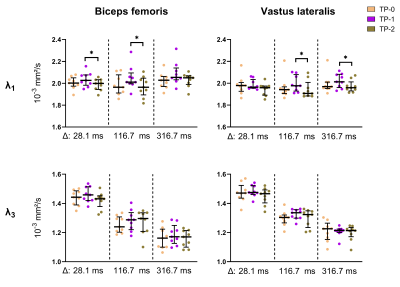
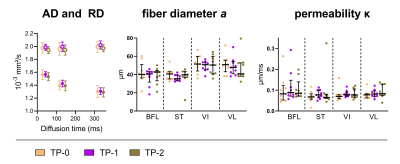
For the conventional DTI parameters, we found an increase in FA and decrease in MD, λ2 and λ3 with increasing diffusion times, but no change in λ1 (p>0.1) (Figures 1-3). The differences between TP-0 – TP-1 and TP-1 – TP-2 increased for longer diffusion times (Figure 2). A similar pattern was seen in the sensitivity analysis, where the largest percentage change for all DTI indices was found between TP-0 and TP-1 for Δ=116.7ms (median: 2.27% range: 0.67-3.29%) followed by Δ=316.7ms (median: 1.39% range: 0.09-3.27%) and lastly Δ=28.1ms (median: 1.19% range: 0.24-1.67%) (Table 1); but only reached significance for MD in the VL muscle (p<0.01). The RPBM parameters revealed a larger fiber diameter in the quadriceps muscles VI and VL compared to the hamstring muscles BFL and ST (p<0.02, Figure 3). No changes in fiber diameter or permeability over time-points were observed. Both RPBM-derived parameters showed a large variability between analyzed muscles and subjects.Discussion
Our results demonstrate the highest sensitivity to micro-trauma at a diffusion time of 116.7ms followed by Δ=316.7ms and thereafter at Δ=28.1ms. This is in line with previous studies investigating time-dependent diffusion in simulated muscle and neuromuscular disease8,9 and confirms the potential of DTI with longer diffusion times for the detection and recovery of muscle micro-trauma. The observed changes in DTI parameters over the diffusion times are also in line with literature9,10. We did not find changes in the RPBM-derived fiber diameter and membrane permeability over time-points. The values for the fiber diameter lie within the range of normal histology values3,11. The permeability values are comparable with published values in calf muscles5. The large variability in the RPBM parameters might be explained by the limited number of diffusion times used. Increasing the range or number of diffusion times might improve the results, but will prolong the scan time.Conclusion
The largest percentage change in DTI indices was found for Δ=116.7ms, suggesting that a diffusion time beyond the typical range of SE DTI can increase the sensitivity to muscle micro-trauma. No changes in RPBM-derived parameters were observed.Acknowledgements
No acknowledgement found.References
1. Reurink G, Brilman EG, de Vos RJ, et al. Magnetic Resonance Imaging in Acute Hamstring Injury: Can We Provide a Return to Play Prognosis? Sport Med. 2014;45(1):133-146. doi:10.1007/s40279-014-0243-1
2. Hooijmans MT, Monte JRC, Froeling M, et al. Quantitative MRI Reveals Microstructural Changes in the Upper Leg Muscles After Running a Marathon. J Magn Reson Imaging. 2020. doi:10.1002/jmri.27106
3. Polgar J, Johnson MA, Weightman D, Appleton D. Data on fibre size in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;19(3):307-318. doi:10.1016/0022-510X(73)90094-4
4. Novikov DS, Fieremans E, Jensen JH, Helpern JA. Random walks with barriers. Nat Phys. 2011;7(6):508-514. doi:10.1038/nphys1936
5. Fieremans E, Novikov DS, Sigmund EE, Liu K, Jensen JH, Helpern JA. In Vivo Measurement of Membrane Permeability and Fiber size in Calf Muscle Using Time-dependent DWI. Proc Intl Soc Mag Reson Med. 2011:1153.
6. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394-406. doi:10.1016/J.NEUROIMAGE.2016.08.016
7. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29(1):196-205. doi:10.1109/TMI.2009.2035616
8. Berry DB, Englund EK, Galinsky V, Frank LR, Ward SR. Varying diffusion time to discriminate between simulated skeletal muscle injury models using stimulated echo diffusion tensor imaging. Magn Reson Med. 2020;(October):1-13. doi:10.1002/mrm.28598
9. McDowell AR, Feiweier T, Muntoni F, Hall MG, Clark CA. Clinically feasible diffusion MRI in muscle: Time dependence and initial findings in Duchenne muscular dystrophy. Magn Reson Med. 2021;(July):1-9. doi:10.1002/mrm.28945
10. Lemberskiy G, Feiweier T, Gyftopoulos S, Axel L, Novikov DS, Fieremans E. Assessment of myofiber microstructure changes due to atrophy and recovery with time-dependent diffusion MRI. NMR Biomed. 2021;34(7):1-11. doi:10.1002/nbm.4534
11. Johnson MA, Sideri G, Weightman D, Appleton D. A comparison of fibre size, fibre type constitution and spatial fibre type distribution in normal human muscle and in muscle from cases of spinal muscular atrophy and from other neuromuscular disorders. J Neurol Sci. 1973;20(4):345-361. doi:10.1016/0022-510X(73)90169-X
Figures