4048
AI assisted upscaling of low-resolution cardiac MRI reduces acquisition time and yields qualitatively comparable images to standard techniques1Diagnostic and interventional radiology, University Hospital Bonn, Bonn, Germany, 2Quantitative Imaging Laboratory Bonn, Bonn, Germany, 3Philips GmbH Market DACH, Hamburg, Germany, 4Philips MR Clinical Science, Best, Netherlands
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Cardiovascular
AI assisted upscaling of low-resolution cine bSSFP images yields comparable image quality to conventional images with no clinically significant difference in volumetric data at a reduction of acquisition time by a factor of 1.5 to 2.
Keywords: Artificial intelligence, acceleration, Superresolution, cardiac MRI
Introduction
Cardiac magnetic resonance imaging (MRI) is a vital tool in a number of pathologies such as acute myocarditis or myocardial infarction. Unfortunately, long acquisition times and confined spaces limit the use of cardiac MRI. Novel imaging techniques offer a solution to one of these problems by reducing acquisition times using undersampling in combination with artificial intelligence (AI) reconstruction. In this study we compared standard ECG-triggered balanced steady state free precession (bSSFP) cine images to ECG triggered low-resolution bSSFP images with AI upscaling, termed superresolution.Methods
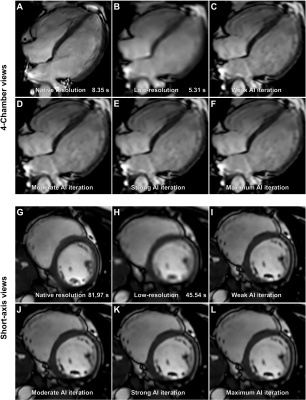
Cardiac MR cine images were acquired in healthy volunteers using standard ECG-triggered bSSFP techniques as well as ECG-triggered low-resolution bSSFP with AI upscaling. Images were reconstructed using a vendor provided prototype (Philips SmartSpeed Precise Image). This AI-based reconstruction technique consists of a series of convolutional neural networks (CNNs): Adaptive-CS-Net (1) allowed to reconstruct images acquired with Compressed SENSE based variable density undersampling patterns. CNN was applied during coil combination, removing the noise and undersampling artifacts from the images in order to obtain good image quality from accelerated acquisitions (2). Subsequently, Precise Image Net, an AI-model, was applied to remove ringing artefacts and to replace the traditional zero-filling strategy to increase the matrix size and therewith the sharpness of the images; these type of networks are known as superresolution networks (3, 4). The network was trained on pairs of low- and high-resolution data with k-space crops to induce ringing. Data consistency checks were implemented to match the resulting k-space with the measured k-space data. The full reconstruction pipeline generates images with improved SNR and sharpness, higher matrix size and reduced ringing artefacts, and can be applied to all 2D cartesian acquisitions. The level of noise reduction was variable with settings ranging from weak, moderate, strong, to maximum. Left ventricular ejection fraction (LVEF), left ventricular end diastolic volume (LVEDV), left ventricular end diastolic volume index (LVEDVi), and interventricular septum thickness at diastole (IVSD) were compared using the repeated measures analysis of variance (ANOVA) between six groups: native resolution, low-resolution, and low-resolution with upscaling and different levels of denoising. Acquisition times were compared using the student’s paired t test. Apparent signal to noise ratios (aSNR) and apparent contrast to noise ratios (aCNR) were calculated as previously described and compared using repeated measures ANOVA (5). Subjective image quality assessment was rated by two radiologists for all short-axis and 4-chamber views for all six data sets on a 5-point rating scale regarding three image criteria: blood-pool to myocardium contrast, endocardial edge definition, and artefacts, as previously described (6). An overall score was determined by the equal weight average of all three criteria: 1 non-diagnostic, 5 excellent.Results
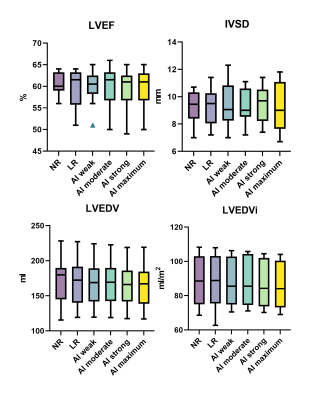
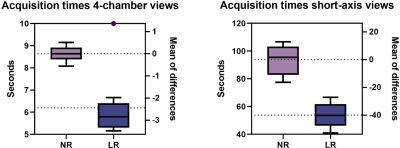
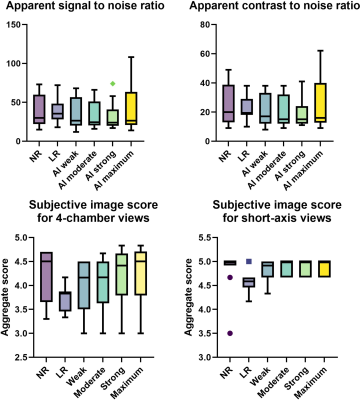
Data from 10 participants was acquired (30.3±3.1 years old, 8 male). Acquisition duration for superresolution acquisitions was significantly shorter than standard acquisitions for 4-chamber views (6.20±1.42 vs 8.64±0.33 s, p=.0003) as well as for short-axis view (53.84±8.47 vs 93.83±10.04 s, p<.0001). Subjective image quality was not significantly different between the groups for 4-chamber views or short-axis views (p=.685 and p=.073). Interclass correlation ranged from excellent for conventional 4-chamber views (0.966, CI 0.891-0.989) to good, for example low-resolution 4-chamber views (0.812, CI 0.403-0.941). No difference was found between the groups regarding aSNR and aCNR (p=.42 and p=.42). Regarding volumetry, no difference was observed between the groups regarding LVEF (p=.64) or IVSD (p=.72). Significant differences in LVEDV were noted between the standard sequence and superresolution with maximum denoising (171.1±32.7 ml vs 164.3±31.2, p=.039), superresolution weak vs superresolution maximum (167.7±31.7 vs 164.3±31.2, p=.0006), superresolution moderate vs superresolution strong (167.8±31.6 vs 165.5±30.8, p=.025), and superresolution moderate vs superresolution maximum (167.8±31.6 vs 164.3±31.2, p=.005).Discussion
AI assisted upscaling led to significantly faster acquisition time averaging about 1.5 up to 2 times faster than conventional techniques without affecting aSNR and aCNR, as a result of improved SNR by lower resolution which can then be sacrificed for higher acceleration factors. While volumetric analysis showed nearly identical results for LVEF and IVSD, LVEDV demonstrated a tendency to decrease going from conventional sequences and low-resolution sequences with maximum AI denoising. One possible reason for this may be progressive denoising at the edge of high contrast areas, such as the blood pool and myocardium, as the denoising algorithm increases, leading to discrete changes in perceived volume. On the other hand, results might be biased due to the small participant group and this phenomenon might disappear as more data sets are analyzed. Clinically, the differences were not relevant with the largest mean difference being 6.7 ml (conventional vs maximum AI) and about 3 ml for the other differences. Although low-resolution images without upscaling did appear unsharp compared to their native resolution and upscaled counterparts, no statistical differences were noted for any groups regarding subjective image quality. This may be due to the fact that low-resolution acquisitions without upscaling scored relatively high for the artefacts category, thus inflating the aggregate subjective image quality score while the other two categories, contrast and edge definition, scored less.Conclusion
Low-resolution AI assisted upscaling of cardiac cine MRI sequences leads to a significant reduction in acquisition times without a significant difference in volumetric results or subjective image quality in healthy volunteer participants.Acknowledgements
No acknowledgement found.References
1. Pezzotti N, Weerdt E de, Yousefi S, Elmahdy MS, van Gemert J, Schülke C et al. Adaptive-CS-Net: FastMRI with Adaptive Intelligence; 13-Dec-19. Available from: URL: https://arxiv.org/pdf/1912.12259.
2. Peeters JM, Chung H, Valvano G, Yakisikli D, van Gemert J, de Weerdt E and van de Ven K. Philips SmartSpeed. No compromise Image quality and speed at your fingertips. Philips SmartSpeed white paper.
3. Dong C, Loy CC, He K, Tang X. Image Super-Resolution Using Deep Convolutional Networks; 31-Dec-14. Available from: URL: https://arxiv.org/pdf/1501.00092.
4. Li Y, Sixou B, Peyrin F. A Review of the Deep Learning Methods for Medical Images Super Resolution Problems. IRBM 2021; 42(2):120–33. Available from: URL: https://www.sciencedirect.com/science/article/pii/S1959031820301408.
5. Bischoff LM, Katemann C, Isaak A, Mesropyan N, Wichtmann B, Kravchenko D et al. T2 Turbo Spin Echo With Compressed Sensing and Propeller Acquisition (Sampling k-Space by Utilizing Rotating Blades) for Fast and Motion Robust Prostate MRI: Comparison With Conventional Acquisition. Investigative Radiology 2022; 00004424-990000000-00049(September 1, 2022):10.1097/RLI.0000000000000923. Available from: URL: https://journals.lww.com/investigativeradiology/Fulltext/9900/T2_Turbo_Spin_Echo_With_Compressed_Sensing_and.49.aspx.
6. Pednekar AS, Wang H, Flamm S, Cheong BY, Muthupillai R. Two-center clinical validation and quantitative assessment of respiratory triggered retrospectively cardiac gated balanced-SSFP cine cardiovascular magnetic resonance imaging in adults. J Cardiovasc Magn Reson 2018; 20(1):44. Available from: URL: https://jcmr-online.biomedcentral.com/articles/10.1186/s12968-018-0467-6.
Figures