4034
DeepGraspT1: Deep Learning-Enabled GRASP T1 Mapping1Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Electrical and Computer Engineering, NYU Tandon School of Engineering, New York, NY, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Machine Learning/Artificial Intelligence
Golden-angle RAdial Sparse Parallel (GRASP) MRI has recently been extended for rapid, accurate and robust T1 mapping (GraspT1) that can be performed during free breathing. However, GraspT1 implements a conventional T1 mapping framework that reconstructs an image series from undersampled dynamic k-space in the first step and then performs pixel-wise parameter fitting in the second step. This leads to a slow and cumbersome pipeline to obtain T1 maps. In this work, we developed deep learning-based GraspT1 (DeepGraspT1), which directly estimates T1 maps from undersampled k-space and enables additional acceleration that outperforms conventional iterative reconstruction.Introduction
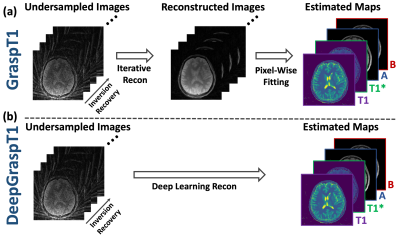
Golden-angle RAdial Sparse Parallel (GRASP) MRI is a fast-imaging technique that can be applied for accelerated free-breathing dynamic imaging1. GRASP combines stack-of-stars golden-angle radial sampling with multi-coil compressed sensing reconstruction, and it has later incorporated a more advanced low-rank subspace model for further improved imaging performance2. GRASP has recently been extended for rapid free-breathing T1 mapping (called GraspT1) using an inversion recovery (IR)-prepared stack-of-stars sequence3, and it has been shown as an accurate and robust technique for fast T1 mapping4. However, like most T1 mapping techniques, GraspT1 employs a two-step quantification pipeline, as shown in Figure 1a. In the first step, IR-prepared images at different inversion times (TIs) are reconstructed from undersampling k-space using constrained iterative reconstruction. From the reconstructed images, T1 maps are then generated in the second step based on pixel-wise fitting. This relatively slow and cumbersome framework represents a major barrier to translate fast quantitative MRI techniques for routine clinical use. One solution to address this challenge is to use deep learning, which has attracted substantial attention in the medical imaging field, including quantitative MRI5. In this work, we propose a deep learning version of GraspT1 called DeepGraspT1, which enables the estimation of T1 maps directly from undersampling k-space, as shown in Figure 1b. The performance of DeepGraspT1 has been demonstrated in T1 mapping of the brain and was compared with standard GraspT1.Methods
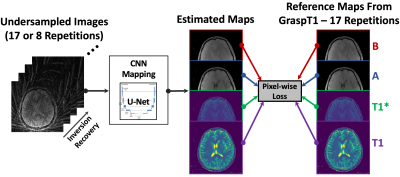
The overall training pipeline for DeepGraspT1 is shown in Figure 2. T1 maps generated from GraspT1 with 17 IR-prepared repetitions are used as the reference for training. Unlike many deep learning-based MRI reconstruction studies that use fully sampled reference for network training, we use results from undersampled images following iterative reconstruction. GraspT1 with 17 IR-prepared repetitions has been shown to ensure sufficient image quality in a prior study4. A total number of 36 brain GraspT1 datasets were used for training. Relevant imaging parameters included: FOV=280x280mm2, matrix size=320x320, spatial resolution=0.875x0.875mm2, slice thickness=3mm, number of slices=32, TR/TE=3.67/1.74ms, flip angle=5o, total acquisition time= 169s.Two specific studies were performed. For the first one, undersampled images with 17 IR-prepared repetitions were used for training, and a neural network was trained to directed estimate 4 parameter maps from undersampled images, including a T1 map, a T1* map, and two additional maps that are generated using a three-parameter T1 fitting model, as described in the reference6. Four L1-norm-based loss functions were enforced between the estimated parameters and the corresponding reference maps from reconstructed GraspT1 images, which were treated as the reference described above. For this study, the hypothesis was that DeepGraspT1 could enable simple and fast T1 estimation without losing accuracy.
For the second study, the references were kept the same, but the input for network training was changed to undersampled images from only 8 IR-prepared repetitions. Golden-angle radial sampling allows us to perform this retrospectively undersampled study. Same as the first study. Four L1-norm-based loss functions were enforced between the estimated parameters and the corresponding reference maps. Standard GraspT1 reconstruction from 8 IR-prepared repetitions was also performed for comparison. This study hypothesized that in addition to an improved imaging pipeline, DeepGraspT1 could also enable additional acceleration that outperforms conventional reconstruction.
For both studies, the trained neural networks were tested in 10 additional GraspT1 datasets that were not included for training. T1 maps obtained using DeepGraspT1 were quantitatively compared with T1 maps obtained from standard GraspT1 with 17 IR-prepared repetitions (as the reference) or 8 IR-prepared repetitions.
Results
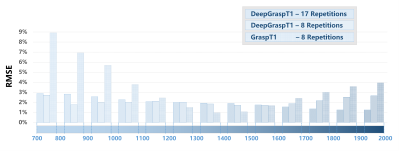
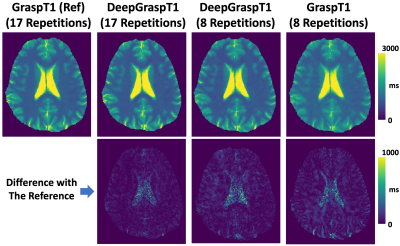
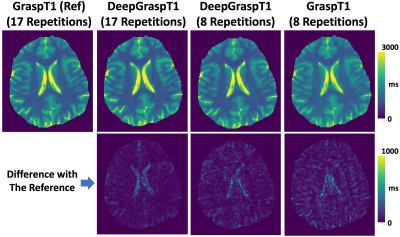
Figure 3 summarizes the T1 values for different studies based on different T1 ranges. First, the results indicate that DeepGraspT1 with both 17 and 8 repetitions enables accurate T1 quantification compared to the reference, with all the RMSE below 5%. Second, the results also suggest that with 8 repetitions, DeepGraspT1 outperforms standard GraspT1 in 9 T1 ranges. The error between T1 estimation between standard GraspT1 with 8 repetitions and reference was up to 7-9% in some T1 ranges.Figure 4 shows T1 maps estimated with different methods in one subject. DeepGraspT1 with both 17 and 8 repetitions, generated good T1 maps compared to the reference. From the error maps, it can be seen that DeepGraspT1 outperforms standard GraspT1 with 8 repetitions, especially in the white matter. Figure 5 shows a similar comparison in another subject, from which similar findings can be obtained.
Conclusion
This work proposed DeepGraspT1, a deep learning version of GraspT1 method for fast and efficient T1 quantification. Compared to standard GraspT1 that requires a two-step reconstruction and fitting scheme, DeepGraspT1 can substantially simplify the processing pipeline while maintaining T1 quantification accuracy, which holds great potential for clinical translation. In the meantime, DeepGraspT1 also enables additional acceleration to further push the imaging speed for T1 mapping.Acknowledgements
This work was supported by the NIH grants R01EB030549, R21EB032917, R21EB031185, R01AR079442 and R01AR081344.References
1. Feng L, Grimm R, Block KT obia., et al.: Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014; 72:707–717.
2. Feng L, Wen Q, Huang C, Tong A, Liu F, Chandarana H: GRASP-Pro: imProving GRASP DCE-MRI through self-calibrating subspace-modeling and contrast phase automation. Magn Reson Med 2020; 83:94–108.
3. Feng L, Liu F, Soultanidis G, et al.: Magnetization-prepared GRASP MRI for rapid 3D T1 mapping and fat/water-separated T1 mapping. Magn Reson Med 2021; 86:97–114.
4. Li Z, Xu X, Yang Y, Feng L: Repeatability and robustness of MP-GRASP T1 mapping. Magn Reson Med 2022; 87:2271–2286.
5. Feng L, Ma D, Liu F: Rapid MR relaxometry using deep learning: An overview of current techniques and emerging trends. NMR Biomed 2022; 35:e4416.
6. Deichmann R, Haase A: Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson 1992; 96:608–612.
Figures