4033
Image sequence partitioning in CRNN-based reconstruction improves ventilation defect detection of 3D-PREFUL with undersampled data
Maximilian Zubke1,2, Filip Klimeš1,2, Andreas Voskrebenzev1,2, Marius Wernz1,2, Till F Kaireit1,2, Agilo L Kern1,2, Marcel Gutberlet1,2, Robin A Müller1,2, Frank Wacker1,2, and Jens Vogel-Claussen1,2
1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany
1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Lung
3D-phase-resolved-functional-lung (3D-PREFUL) MRI enables a non-contrast-enhanced detection of lung ventilation defects. Convolutional, recurrent neural networks (CRNN) can accelerate data acquisition by reconstructing dynamic lung images from undersampled data. However, a remaining bias in reconstruction led to incorrect detection of ventilation defects. Reducing the number of respiratory phases per reconstruction step improved the accuracy of the defect detection. This improvement is demonstrated by comparison of 2 complementary ventilation defect metrics derived from the original data and the 2x, 4x and 6x undersampled data of Asthma, COPD and post-COVID-19 patients reconstructed from a CRNN in partitions of 30,20 and 10 respiratory phases.Introduction
3D-phase-resolved-functional-lung (3D-PREFUL) MRI1 enables radiation-free and non-contrast-enhanced functional lung imaging. As shown in Figure1, a 3D-PREFUL record consists of N respiratory phases for M slices. For each slice, ventilation defects are derived from a sequence of 128x128xN images. A binary map (VD-map) visualizes the ventilation defects per slice. Finally, the total proportion of defects is summarized by the ventilation-defect-percentage (VDP) value.Recently, a convolutional, recurrent neural network (CRNN) trained to reconstruct undersampled 128x128x30 3D-PREFUL image sequences showed promising results2. However, CRNN-based reconstruction of 30 respiratory phases is accompanied by a bias resulting in incorrect ventilation defect detection. Since the CRNN requires a fixed data shape of 128x128xZ, image sequences of 128x128xN, with N>Z, need to be subdivided into multiple partitions, which are reconstructed by the CRNN and afterwards concatenated back to the original sequence. Figure2 illustrates this process.
In this study, Z=10, 20 and 30 were examined for 2x, 4x and 6x artificially undersampled 3 data regarding their respective impact on the accuracy of VD-maps.
Methods
The artificial undersampled data originating from 3D-PREFUL data1 were reconstructed as described in Klimes et al.1 and were artificially undersampled as described by Zubke et.al.2. 4x undersampled means, that only 25% of original k-space data were available.Overall, 9 CRNNs were trained to reconstruct the aforementioned combinations of Z and undersampling levels. Training data were provided by 30 healthy volunteers (female=5, age-range: 22-71). The evaluation was conducted with 3D-PREFUL-MRI scans from 13 Asthma (female=5, age-range: 35-61), 13 COPD (female=9, age-range: 47-74) and 13 post-COVID-19 (female=4, age-range: 23-69) patients plus a group of 13 healthy volunteers (female=7, age-range: 18-65).
After reconstruction (see Figure2), Regional-Ventilation3 (RVent) metric and the resulting ventilation defect detection were evaluated for each combination of Z and undersampling level. RVent was evaluated by median difference, the defect detection was measured by mean difference of the VDP-value and the Dice coefficient of VD-maps compared to the ground truth (GT). Additionally, paired Wilcoxon-tests were performed to detect significant differences between each Z-value and GT as well as between the different Z-values per undersampling level. The GT was given by the original 3D-PREFUL measurements.
For comparison, an alternative to RVent, called Flow-Volume-Loop-cross-correlation4 (FVL-CC), was evaluated in the same way.
Results
In 2x undersampled data, no notable effect from Z=30 to Z=20 sequence partitioning was found. Thus, investigation of Z=10 was omitted for this undersampling level.For 4x undersampled data, median value of RVent in Z=30 partitioned data does not differ significantly in comparison to GT. However, the Dice coefficient of corresponding VD-maps from Z=30 data and Z=20 data differs significantly (p<0.005) in all cohorts. Reconstruction with Z=20 decreased the median distance to the perfect Dice coefficient (1.0) from 0.161(0.140 - 0.173) to 0.085(0.082 - 0.101) for Asthma, from 0.140(0.101 - 0.177) to 0.079(0.069 - 0.107) for post-COVID-19 and from 0.152(0.125 - 0.156) to 0.080(0.068 - 0.098) for COPD-patients.
In contrast to RVent, the median value of FVL-CC of Z=30 partitioned data differs significantly (p<0.005) from the GT. Z=20 and Z=10 data do not differ significantly from GT. Additionally, also the VD-map resulting from FVL-CC differs significantly (p<0.005) between Z=30 and Z=20 partitioned data. Consequently, Z=20 increased the accuracy of VD-maps similar as for RVent.
For FVL-CC, the reconstruction using Z=20 decreased the median distance from 0.161(0.140 - 0.173) to 0.085(0.082 - 0.101) for Asthma, from 0.140(0.101 - 0.177) to 0.079(0.069 - 0.107) for post-COVID-19 and from 0.152(0.125 - 0.156) to 0.080(0.068 - 0.098) for COPD-patients. Moving from Z=20 to Z=10 continues this trend for both metrics, but with a lower advancement than switching from Z=30 to Z=20.
Finally, as shown in Figure3, only Z<=20 enables ventilation defect localization with a Dice coefficient close to 90%, from 4x undersampled MR-data. At this undersampling level, Z=20 leads to a Dice-improvement of RVent-based VD-Map up to 12.6% for Asthma, 11.4% for COPD and 13.9% for COVID-19 cases. At FVL-CC, Dice-improvements up to 26% ,18.6% and 27.3% were measured. Figure4 illustrates these improvements by sample VD-maps.
Beyond the Dice coefficient of VD-maps, reducing Z also decreases the distance of RVent and FVL-CC median values as well as VDP-values to corresponding values from the GT.
Same behavior for 6x undersampled data. However, more undersampling leads to diminished improvements. The analysis of healthy volunteers showed the same effects and presented no negative side-effects like a reduced recognition of healthy lung parenchyma.
Table1 shows further distances of the four cohorts to GT of both involved ventilation metrics.
Discussion
The error observed in this study when high Z values are used can be explained by so called long-term dependencies of the pixel signal over time, which is a known weakness of recurrent network architectures5. In context of 3D-PREFUL, this weakness can be addressed using smaller Z values for sequence partitioning. Since the used collectives are quite small, the power behind the significance values is weak. Nevertheless, the trends indicated by the p-values were confirmed by further metrics.Conclusions
Summarized, decreasing Z of a CRNN from 30 to 10 increases the accuracy of MR-based ventilation defect localization in 4x and 6x undersampled MR-data measured by two complement metrics. This enables a detection of Asthma-, COPD- and post-COVID-19-related ventilation defects from 4x undersampled data with a Dice coefficient of minimum 90%.Acknowledgements
No acknowledgement found.References
- Klimeš F, Voskrebenzev A, Gutberlet M et.al. 3D phase‐resolved functional lung ventilation MR imaging in healthy volunteers and patients with chronic pulmonary disease. Magnetic Resonance in Medicine. 2021; 85(2), 912-925. doi: 10.1002/mrm.28482
- Zubke M, Klimeš F, Voskrebenzev A. et.al. Deep Learning based image reconstruction for accelerated 3D phase-resolved functional lung (PREFUL) ventilation assessment of post-COVID-19 patients from undersampled MRI images. ISMRM Annual Meeting. 2022
- Klimeš F, Voskrebenzev A, Gutberlet M. et.al. Free-breathing quantification of regional ventilation derived by phase-resolved functional lung (PREFUL) MRI. NMR in Biomedicine. 2019; 32(6), e4088. doi: 10.1002/nbm.4088
- Voskrebenzev A, Gutberlet M, Klimeš F. et.al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magnetic Resonance in Medicine. 2018;79(4), 2306-2314. doi: 10.1002/mrm.26893
- Bengio Y, Frasconi P, Simard P. The problem of learning long-term dependencies in recurrent networks. IEEE International Conference on Neural Networks. 1993;3, 1183-1188. doi: 10.1109/ICNN.1993.298725
Figures

Figure1: A 3D-PREFUL-MRI record consists of M series of Nx128x128 images each. Each series shows a different coronal body slice. For each slice, a VD-Map is calculated. The VDP-value summarizes the amount of ventilation defects over all slices.
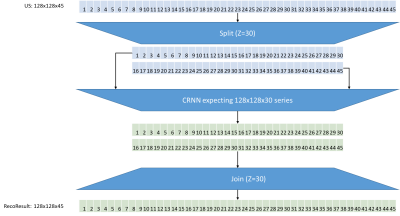
Figure2: An undersampled image sequence of 128×128×N is divided into n partitions of length Z. For example, with Z=30 a sequence of 45×128×128 images is divided into 2 subsequent partitions of 30×128×128 images. If N mod Z >0, the last partition starts at (N-Z) and ends at N. Next, each partition is reconstructed by a CRNN trained to reconstruct image sequences with shape 128×128×Z. Finally, the reconstructed partitions of length Z are concatenated back to the original sequence shape having length N.
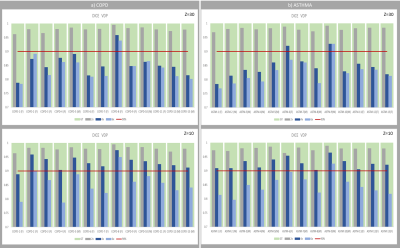
Figure3: Dice coefficient of ventilation defects from COPD (a) and Asthma(b) patients. Reducing Z from 30 to 10 leads to improved Dice coefficient at 4x and 6x undersampled data. At 4x undersampling, modified reconstruction ensures a Dice coefficient >= 90%.
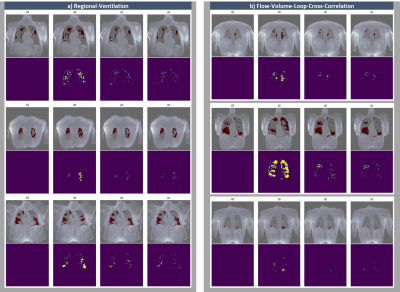
Figure4: Comparison of detected defects (red) and error maps to ground truth (GT) below with decreasing Z from left to right for regional-ventilation (a) and flow-volume-loop-cross-correlation (b).
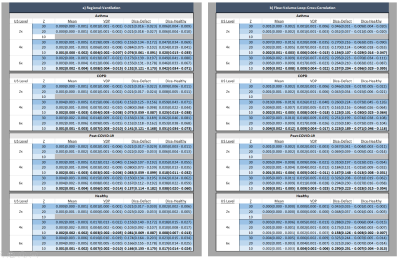
Table1: Distance to ground truth of both ventilation metrics and Dice coefficient of resulting VD-maps. Best result per ventilation metric at undersampling level bold.
DOI: https://doi.org/10.58530/2023/4033