4020
Zero-echo time (ZTE) pulse sequence enables EEG-fMRI with suppression of susceptibility artifacts.
Ayako Imamura1,2,3, Rikita Araki4, Yukari Takahashi3, Koichi Miyatake2, Fusao Kato3, Sakiko Honjoh2, and Tomokazu Tsurugizawa3,5,6
1Ph. D. Program in Humanics, University of Tsukuba, Tsukuba, Japan, 2International Institute for Integrative Sleep Medicine (WPI-IIIS), University of Tsukuba, Tsukuba, Japan, 3Department of Neuroscience, The Jikei University School of Medicine, Tokyo, Japan, 4Bruker Japan K.K., Yokohama, Japan, 5Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 6Faculty of Engineering,, University of Tsukuba, Tsukuba, Japan
1Ph. D. Program in Humanics, University of Tsukuba, Tsukuba, Japan, 2International Institute for Integrative Sleep Medicine (WPI-IIIS), University of Tsukuba, Tsukuba, Japan, 3Department of Neuroscience, The Jikei University School of Medicine, Tokyo, Japan, 4Bruker Japan K.K., Yokohama, Japan, 5Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 6Faculty of Engineering,, University of Tsukuba, Tsukuba, Japan
Synopsis
Keywords: fMRI, fMRI
Simultaneous recording of fMRI and EEG is a promising approach to realize functional brain imaging with high temporal and spatial resolution. However, using standard echo planar imaging in conventional fMRI studies, implantation of electrodes on the cortical surface induces strong magnetic susceptibility artifacts in fMRI images. The zero-echo time (ZTE) sequences show a remarkable reduction in sensitivity to magnetic susceptibility artifacts and motion-derived artifacts. In this study, we showed that the ZTE sequence suppressed the susceptibility artifact by electrodes and air in the ear canal in mice. Furthermore, ZTE showed the typical functional connectivity in the mice at resting state.Introduction
The combination of functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) (EEG-fMRI) is a promising technique for functional brain imaging technique in neuroscience, compensating for the low temporal resolution of fMRI. However, when using standard echo planar imaging (EPI) in conventional fMRI studies, implantation of electrodes on the cortical surface induces strong magnetic susceptibility differences in the brain, resulting in severe signal dropout and strong geometric distortion [1]. MB-SWIFT [2,3] and zero-echo time (ZTE) [4] sequences show a remarkable reduction in sensitivity to magnetic susceptibility artifacts, motion-induced artifacts, and acoustic noise in rats and/or humans. However, there are no studies of ZTE sequence that have evaluated mouse fMRI or susceptibility artifacts by electrodes in mice. The purpose of this study is to assess the feasibility of ZTE for the reduction of susceptibility artifacts by electrodes and to investigate functional connectivity at the resting state in mice.Methods
AnimalsSix C57BL/6 male mice (body weight 28 –38 g) were used for the experiments. Mice were maintained in a temperature-controlled environment on a 12h/12h light/dark cycle.
Electrodes implantation surgery
Mice were anesthetized with isoflurane (2% for induction; 1-1.5% for maintenance) and implanted with L-shaped bent tungsten electrodes in the burr holes of the skull above the frontal cortex, cerebellum, and the olfactory bulb. Protocols were approved by the institutional animal care and use committee.
fMRI experiment
The MRI experiments were conducted on a Bruker 9.4T scanner with a 4ch brain array coil. MRI experiment was performed with 1% isoflurane in air. The respiratory movement was monitored, and the body temperature was maintained at 36 °C by circulating hot water during the measurement. fMRI images with spin-echo EPI (SE-EPI) sequence were acquired with the following parameters, TR/TE = 3000/20 ms, spatial resolution = 150 x 150 x 500 μm3 / voxel, 15 slices, for 15 min (300 volumes). fMRI images with ZTE sequence were acquired with the following parameters, TR = 3000 ms, flip angle = 1 degree, spatial resolution = 230 x 230 x 781 μm3 / voxel, for 15 min (300 volumes). Anatomical images were acquired for spatial correction using multi-slice rapid acquisition with relaxation enhancement (RARE) following parameters: TR/effective TE = 2500/48 ms, spatial resolution = 59 x 59 x 500 μm3 / voxel, RARE factor = 8, 4 averages.
Image processing
For preprocessing, the slice timing correction, spatial realignment, normalization, and smoothing were performed by SPM12. Averaged signals in the cerebrospinal fluid, the white matter, and the head motion parameters were used as nuisance regressors. Detrending and temporal filtering (0.008 – 0.08 Hz) were then performed using CONN toolbox. The voxel-wise temporal signal-to-noise ratio (tSNR) was calculated by dividing the temporal mean by the temporal standard deviation throughout 300 volumes within each voxel. Independent component analysis (ICA) was performed with Group ICA of fMRI Toolbox (GIFT) software. First, all data sets underwent a subject-specific principal component analysis (PCA), which estimated 150 components. All subjects’ reduced data sets were concatenated and underwent a PCA that estimated 40 components at the group level. Subsequently, group-level spatial ICA was performed on the PCA output, identifying 40 functional components.
Results & Discussion
The ZTE image showed no signal loss derived from the tungsten electrode, while a severe signal loss was observed in SE-EPI (Fig. 1). Additionally, the susceptibility artifact by the air in the ear canal was not observed in the ZTE image. The tSNR as well as the ICA were then performed to assess the feasibility of ZTE for resting state fMRI in naïve mice because tSNR gives information on the data quality of fMRI time series. The tSNR map showed a similar range of tSNR between SE-EPI and ZTE, and a more uniform tSNR in the upper part of the ZTE compared to the SE-EPI (Fig.2A). The averaged tSNR in the cortex was not significantly different between ZTE and SE-EPI (Fig.2B). ICA shows the typical functional connectivity, such as default mode network (DMN), which is anterior-posterior connectivity along the axis of the cingulate and retrosplenial cortices, lateral cortical network (LCN), bilateral secondary somatosensory cortex network (S2), local network within thalamic nuclei (thalamus) in SE-EPI and ZTE sequence (Fig.3). These results optimize the feasibility of ZTE sequence for the resting state fMRI. Furthermore, because ZTE sequence suppresses the susceptibility artifact by the electrodes, it is useful for the simultaneous recording of fMRI and EEG.Acknowledgements
No acknowledgment was found.References
- In, Myung-Ho, et al. "Correction of metal-induced susceptibility artifacts for functional MRI during deep brain stimulation." NeuroImage 158 (2017): 26-36.
- Lehto, Lauri J., et al. "MB-SWIFT functional MRI during deep brain stimulation in rats." Neuroimage 159 (2017): 443-448.
- Paasonen, Jaakko, et al. "Multi-band SWIFT enables quiet and artefact-free EEG-fMRI and awake fMRI studies in rat." NeuroImage 206 (2020): 116338.
- Ljungberg, Emil, et al. "Silent zero TE MR neuroimaging: Current state-of-the-art and future directions." Progress in Nuclear Magnetic Resonance Spectroscopy 123 (2021): 73-93.
Figures
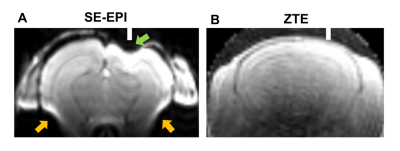
Figure.1 : Images of a mouse brain with implanted Tungsten electrodes by SE-EPI and ZTE sequencing. Green arrow indicates the signal loss by electrode. Orange arrows indicate the signal loss by the air in the ear canal. White line shows the position of electrode.
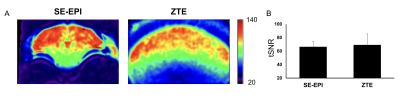
Figure.2 : (A)The tSNR map of SE-EPI and ZTE images and (B) averaged tSNR in the cortex in SE-EPI and ZTE sequence.

Figure.3 : ICA analysis of SE-EPI and ZTE sequence. Cg, cingulate; DMN, default mode network; LCN, lateral cortical network; RSC, retrosplenial cortex; S2, secondary somatosensory cortex
DOI: https://doi.org/10.58530/2023/4020