4015
Motion-corrected quantification of cerebral venous oxygenation in vulnerable populations: from neonates to older adults1Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States, 2Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Center for Imaging Science, Johns Hopkins University Whiting School of Engineering, Baltimore, MD, United States, 5Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 6Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Keywords: Oxygenation, Oxygenation
Cerebral venous oxygenation (Yv) is a biomarker in various brain diseases such as hypoxic-ischemic-encephalopathy in neonates and Alzheimer's disease in the elderly. T2-Relaxation-Under-Spin-Tagging (TRUST) MRI is a widely used technique to measure global Yv level. However, subject motion during TRUST scan can cause considerable errors in Yv quantification. This work developed an automatic algorithm for motion-corrected quantification of Yv, which showed improved precision of Yv estimation in both neonates and elderly adults.INTRODUCTION
Cerebral venous oxygenation (Yv) is an important physiological parameter for the brain’s oxygen utilization and has been suggested to be a biomarker in various brain diseases, ranging from hypoxic-ischemic-encephalopathy in neonates1,2 to Alzheimer's disease in the elderly.3,4 T2-Relaxation-Under-Spin-Tagging (TRUST) MRI is a widely used technique to measure global Yv level, and has been validated against gold-standard 15O-PET.5 Although TRUST has a short scan time (1.2min), for noncompliant subjects such as neonates and older adults, subject motion can still cause considerable errors in Yv quantification.6 The goal of this work is to develop an automatic algorithm to exclude motion-corrupted images from TRUST Yv estimation. This algorithm was developed using datasets of non-sedated healthy neonates (N=49), which contains considerable motion. The generalizability of this algorithm was validated on datasets of elderly adults (N=223).METHODS
TRUST MRI for Yv measurement: The TRUST MRI technique is based on the well-established relationship between blood T2 and Yv.7 Briefly, TRUST uses subtraction between control and venous-labeled images to yield difference images, in which the tissues are cancelled out, leaving only the pure blood signal in the superior-sagittal-sinus (SSS). Venous blood T2 is quantified by using T2-preparation with varying effective-TEs (eTEs) and then converted to Yv using a calibration model.7Experiments:
Neonates: We collected TRUST data from 33 healthy neonates (Table 1), among which 14 neonates underwent 2-3 TRUST scans, yielding a total of 49 TRUST scans. All neonates were scanned in natural sleep without sedation on a Siemens 3T scanner. TRUST used the following parameters:8 field-of-view (FOV)=160×160mm2, voxel-size=2.5×2.5×5.0mm3, TR/TI=3000ms/1020ms, 4 eTEs of 0,40,80,160ms, 3 dynamics for each eTE, and duration=1.2min.
Older adults: 233 TRUST scans were acquired from a group of older adults, as part of the BIOCARD study (Table 1).9 Each subject underwent one TRUST scan on a Philips 3T system. The TRUST sequence parameters were similar to those in neonates except voxel-size=3.4×3.4×5.0mm3 and FOV=220×220mm2.
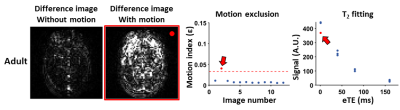
Motion detection and correction algorithm: Each TRUST scan yields 12 difference images, consisting of 3 images at each of the 4 eTEs (Figure 1). A motion-free difference image should contain only a few bright pixels in veins such as SSS (Figure 1, yellow arrow); while a motion-contaminated difference image (Figure 1, red-highlighted) will contain considerable residual tissue signal. Therefore, the motion detection algorithm is based on the amount of tissue signals in difference images, as described below.
The TRUST images were preprocessed using SPM realignment.10 Pairwise subtraction between control and labeled images was conducted to yield difference images. Then, the algorithm was divided into two major steps. The first step was to automatically compute a tissue mask for each dataset, which is important for the determination of tissue signals in the difference images. This step used a label and difference image that has minimal motion, and is illustrated in Figure 2A.
The second major step is to calculate the tissue signal for each of the difference images. As shown in Figure 2B, we used the tissue mask to calculate a motion index ε for each difference image. ε was defined as the mean absolute intensity of the masked difference image normalized by the averaged signal in the masked eTE0 label image. Difference images with large ε were excluded, where the optimal threshold for ε was determined using neonatal data (see below). The blood T2 and Yv were estimated from the remaining images following the literature.5,8 The uncertainty of Yv estimation, ΔR2, was computed as the width of 95% confidence-interval of the fitted 1/T2.
Study 1: The optimal ε threshold was selected using the neonatal data by maximizing the Dice-coefficient between the automatic detection of motion-contaminated images and manual identification, which served as a gold-standard. Manual identification was performed by an experienced researcher (DJ, 10-year experience). Yv and ΔR2 values computed with and without automatic motion exclusion were compared using Wilcoxon signed-rank tests. In the 14 neonates with multiple TRUST scans, the test-retest coefficient-of-variation (CoV) of Yv was also calculated and compared.
Study 2: The algorithm optimized in Study 1 was applied to the elderly datasets to evaluate its generalizability.
RESULTS AND DISCUSSION
Study 1: The optimal ε threshold was determined to be 0.033. At this threshold, the Dice-coefficient was 0.895 compared to manual identification. Figure 3A shows representative neonatal data and the automatically detected motion-corrupted images.Across all neonatal data, compared to no motion exclusion, the algorithm yielded a lower ΔR2 (4.2±1.8Hz vs. 7.6±8.6Hz, P=0.0002, Figure 3C), while the mean Yv showed no difference (65.5±4.8% vs. 65.4±7.1%, P=0.65, Figure 3B). In the 14 neonates with multiple TRUST scans, the algorithm gave a lower CoV (3.4±3.3% vs. 8.5±5.4%, P=0.001, Figure 3D).
Study 2: Figure 4 displays the data of an elderly subject with motion contamination. Across the 223 scans, the algorithm yielded a Dice-coefficient of 0.824 compared with manual identification. There was no significant difference in ΔR2 (P=0.88) computed with and without motion exclusion, since motion is rare in the adult data – only 10 out of the 2676 difference images (223 scans×12 images/scan) were manually identified as motion-contaminated.
CONCLUSION
We have developed an automatic motion exclusion algorithm to improve the precision of Yv quantification in motion-prone subjects such as neonates and older adults.Acknowledgements
No acknowledgement found.References
1. Shetty AN, Lucke AM, Liu P, Sanz Cortes M, Hagan JL, Chu ZD, Hunter JV, Lu H, Lee W, Kaiser JR. Cerebral oxygen metabolism during and after therapeutic hypothermia in neonatal hypoxic-ischemic encephalopathy: a feasibility study using magnetic resonance imaging. Pediatr Radiol 2019;49:224-233.
2. De Vis JB, Petersen ET, Alderliesten T, Groenendaal F, de Vries LS, van Bel F, Benders MJNL, Hendrikse J. Non-invasive MRI measurements of venous oxygenation, oxygen extraction fraction and oxygen consumption in neonates. Neuroimage 2014;95:185-192.
3. Thomas BP, Sheng M, Tseng BY, Tarumi T, Martin-Cook K, Womack KB, Cullum MC, Levine BD, Zhang R, Lu H. Reduced global brain metabolism but maintained vascular function in amnestic mild cognitive impairment. J Cereb Blood Flow Metab 2017;37:1508-1516.
4. Jiang DR, Lin ZX, Liu PY et al. Brain Oxygen Extraction Is Differentially Altered by Alzheimer's and Vascular Diseases. J Magn Reson Imaging 2020;52:1829-1837.
5. Jiang D, Deng S, Franklin CG, O'Boyle M, Zhang W, Heyl BL, Pan L, Jerabek PA, Fox PT, Lu H. Validation of T2 -based oxygen extraction fraction measurement with (15) O positron emission tomography. Magn Reson Med 2021;85:290-297.
6. Stout JN, Tisdall MD, McDaniel P, Gagoski B, Bolar DS, Grant PE, Adalsteinsson E. Assessing the effects of subject motion on T2 relaxation under spin tagging (TRUST) cerebral oxygenation measurements using volume navigators. Magn Reson Med 2017;78:2283-2289.
7. Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med 2012;67:42-49.
8. Liu P, Parkinson C, Jiang D, Ouyang M, De Vis JB, Northington FJ, Tekes A, Huang H, Huisman T, Golden WC. Characterization of MRI techniques to assess neonatal brain oxygenation and blood flow. NMR Biomed 2019;32:e4103.
9. Lin Z, Sur S, Soldan A et al. Brain Oxygen Extraction by Using MRI in Older Individuals: Relationship to Apolipoprotein E Genotype and Amyloid Burden. Radiology 2019;292:140-148.
10. Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical parametric mapping: the analysis of functional brain images: Elsevier; 2011.
Figures
Table 1: Characteristics of the participants
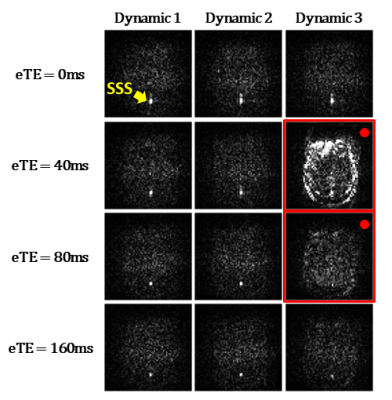
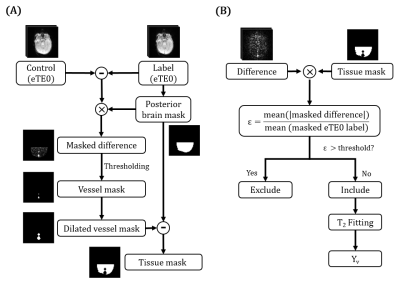

Figure 3: Summary of neonatal results. (A) Representative neonatal data with motion contamination. The red dashed line indicates the threshold for ε. The Yv and ΔR2 were 67.9% and 3.3Hz with motion exclusion, and 80.5% and 12.4Hz without exclusion. Comparison of (B) Yv, (C) ΔR2 and (D) CoV computed with and without motion exclusion across all neonatal data.