4006
Fast 3D free-breathing simultaneous T1 and T1ρ mapping for noncontrast myocardial tissue characterization at 3T1ShanghaiTech University, Shanghai, China, 2Shanghai Clinical Research and Trial Center, Shanghai, China, 3United Imaging Healthcare, Shanghai, China, 4UIH America, Inc., Houston, TX, United States, 5School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Keywords: Myocardium, Tissue Characterization
Relaxation parameters T1 and T1ρ have shown promising results for endogenous assessment of myocardial tissue. However, mapping of the whole moving heart is challenging. This study proposes a novel free-breathing joint T1 and T1ρ mapping technique with near-isotropic spatial resolution, whole left ventricle coverage and short acquisition time of 5-6min. A diaphragmatic navigator was leveraged for prospective motion correction and retrospective motion mitigation. The T1ρ preparation module was optimized to be robust to field inhomogeneities and applicable to 3T. Phantom results indicated good accuracy of the proposed technique, and in vivo feasibility was demonstrated in healthy subjects.Introduction
Quantitative parameter mapping plays an important role in detecting alternations in the myocardium caused by various cardiac diseases 1. Native T1 is a well-recognized biomarker for a range of cardiomyopathies, while T1ρ is an exogeneous contrast for myocardial fibrosis 2. A 2D cardiac MR fingerprinting technique has been developed for simultaneous quantification of T1, T2 and T1ρ, which however, requires breath-hold of 16 heartbeats 3. A free-breathing 3D joint myocardial T1/T1ρ mapping technique 4 has shown promising results at 1.5T by employing an image navigator 5 for respiratory motion correction. In this study, we sought to propose a fast free-breathing 3D myocardial T1 and T1ρ mapping technique, using the widely available diaphragmatic navigator 6 for prospective motion tracking and retrospective motion mitigation with a soft-gating technique 7. Furthermore, the T1ρ preparation module is specifically optimized to be robust to field inhomogeneities to make the technique applicable to 3T.Methods
The diagram of the ECG-triggered free-breathing sequence is shown in Fig. 1, which is a repetitive acquisition of eight cardiac cycles with inversion recovery (IR) and T1ρ preparation (T1ρ prep) pulses with different spin-lock times (TSL) to induce varying T1 and T1ρ contrasts. The excitation pulse in the T1ρ prep is an optimized tan/tanh adiabatic half-passage pulse to make this module robust to field inhomogeneities. A diaphragmatic respiratory navigator (dNAV) 6 is acquired in each heartbeat without rejecting data. To avoid the influence of the preparation pulses on the dNAV signal, a slice-selective IR pulse is applied at the same imaging plane of the dNAV along with the non-selective IR pulse, and the dNAV is played before the T1ρ prep. Cartesian sampling with 4-5x variable-density undersampling 8,9 is used for acceleration.Motion corrected reconstruction
No data was rejected by the dNav to improve the imaging efficiency. To further correct the respiratory motion, a soft-gating technique 7 was adopted to weight the k-space according to the dNav estimated respiratory displacement from the end-expiration bin, where the end-expiration bin was selected to include around 60% of all the acquired data. The motion-weighted data was used for the multi-contrast reconstruction:$$argmin_{x}||W(Ex-y)||_2^2+\lambda\sum_p||T_{p}(x)||_{*}$$
where x is the multi-contrast images to be reconstructed, y is the undersampled k-space data, E is the encoding operator and W is the soft-gating weight. The multi-contrast patch-based locally low-rank regularization 10 was adopted with Tp selecting the local and non-local image patches around pixel p and λ is the regularization parameter.
Parameter quantification
Dictionary matching was performed to quantify T1 and T1ρ from the multi-contrast images efficiently. The dictionary was generated for a range of T1 and T1ρ values for each subject with Bloch simulation, where the relaxation effects during the adiabatic excitation pulses in the T1ρ prep were considered. The signal of the data within 15% of central k-space was averaged as the theoretical signal for each contrast.
Experiments
All imaging experiments were conducted in a 3T United Imaging MR scanner. The phantoms made of different concentrations of agarose and gadolinium contrast were imaged using the IR spin echo and T1ρ prep gradient echo techniques 11 for reference values. The 3D mapping sequence was performed with simulated heart rates from 50bpm to 100bpm with a step of 10bpm and other imaging parameters same to the in vivo imaging.
Four healthy subjects were recruited to test the 3D mapping technique with FOV=320×300×120mm, voxel size=2×2×3mm, short-axis orientation, TR/TE=3.74/1.67ms, flip angle=5°, TI1/TI2=130/210ms, TSL=30/50ms, spin lock frequency=350Hz, number of segments=30. The 2D breath-hold MOLLI 12 and T1ρ-bSSFP 13 were performed for comparison.
Results
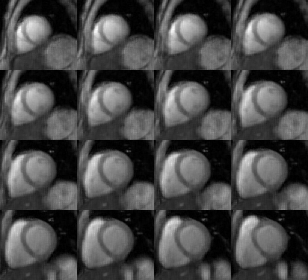
Figure 2 shows the phantom mapping results. Compared with the mapping reference, the proposed technique provided accurate T1 and T1ρ estimations for the simulated range of heart rates with slight overestimation of long T1 and T1ρ at high heart rates. The acquisition time of the proposed technique in heathy subjects is 5.0±0.6min. Representative 3D T1 and T1ρ maps are shown in Fig. 3, which are overall homogeneous. The corresponding eight-contrast images are provided in Fig. 4. The 3D maps of another subject are compared with the 2D breath-hold maps in Fig. 5, where lower T1 was observed for the lateral region at the apex for the 3D free-breathing technique, which may be caused by residual respiratory motion or inhomogeneous B1 field. The septal T1 and T1ρ with the proposed technique and breath-hold MOLLI, T1ρ-bSSFP are comparable (T1: 1189.9±7.5ms vs. 1129.7±4.1ms; T1ρ: 46.9±1.9ms vs. 48.2±2.7ms).Discussion
A fast 3D free-breathing joint T1 and T1ρ mapping technique was developed and validated in phantoms and preliminary healthy subjects. The phantom results indicate the good accuracy and the promising in vivo results suggest the feasibility of the proposed technique. Future work will be focused on improving the respiratory motion correction and accounting for the B1 inhomogeneity in the parameter estimation.Acknowledgements
No acknowledgement found.References
1. Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19(1):75.
2. Han Y, Liimatainen T, Gorman RC, Witschey WR. Assessing Myocardial Disease Using T1rho MRI. Curr Cardiovasc Imaging Rep 2014;7(2):9248.
3. Velasco C, Cruz G, Lavin B, Hua A, Fotaki A, Botnar RM, Prieto C. Simultaneous T1 , T2 , and T1rho cardiac magnetic resonance fingerprinting for contrast agent-free myocardial tissue characterization. Magn Reson Med 2022;87(4):1992-2002.
4. Crabb M, Kunze K, Velasco C, Fotaki A, Munoz C, Hua A, Neji R, Prieto C, Botnar RM. 3D joint T1/T1ρ mapping and water-fat imaging for contrast-agent free myocardial tissue characterization. Proc. Intl. Soc. Mag. Reson. Med. 30 (2022); London, UK.
5. Henningsson M, Koken P, Stehning C, Razavi R, Prieto C, Botnar RM. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med 2012;67(2):437-445.
6. Stuber M, Botnar RM, Danias PG, Kissinger KV, Manning WJ. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology 1999;212(2):579-587.
7. Han F, Zhou Z, Han E, Gao Y, Nguyen KL, Finn JP, Hu P. Self-gated 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) using rotating cartesian K-space (ROCK): Validation in children with congenital heart disease. Magn Reson Med 2017;78(2):472-483.
8. Cheng JY, Zhang T, Ruangwattanapaisarn N, Alley MT, Uecker M, Pauly JM, Lustig M, Vasanawala SS. Free-breathing pediatric MRI with nonrigid motion correction and acceleration. J Magn Reson Imaging 2015;42(2):407-420.
9. Prieto C, Doneva M, Usman M, Henningsson M, Greil G, Schaeffter T, Botnar RM. Highly Efficient Respiratory Motion Compensated Free-Breathing Coronary MRA Using Golden-Step Cartesian Acquisition. J Magn Reson Imaging 2015;41(3):738-746.
10. Bustin A, Lima da Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn Reson Med 2019;81(6):3705-3719.
11. Qi H, Bustin A, Kuestner T, Hajhosseiny R, Cruz G, Kunze K, Neji R, Botnar RM, Prieto C. Respiratory motion-compensated high-resolution 3D whole-heart T1rho mapping. J Cardiovasc Magn Reson 2020;22(1):12.
12. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T-1 mapping of the heart. Magnetic Resonance in Medicine 2004;52(1):141-146.
13. Qi H, Lv Z, Hu J, Xu J, Botnar R, Prieto C, Hu P. Accelerated 3D free-breathing high-resolution myocardial T1rho mapping at 3 Tesla. Magn Reson Med 2022;88(6):2520-2531.
Figures