3999
Navigator acceptance window width does not affect accuracy and precision in free-breathing 2D cardiac MR fingerprinting of the myocardium at 3T.1Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2Cardiology Service, Cardiovascular Department, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, Lausanne, Switzerland, 3Cardiology Service, Department of Medicine, Geneva University Hospital and University of Geneva, Switzerland, Geneva, Switzerland, 4CIBM, Lausanne, Switzerland
Synopsis
Keywords: Myocardium, Relaxometry
We implemented and characterized a free-breathing 2D cardiac joint T1-T2 MR fingerprinting technique at 3T named PARMA that includes a lung-liver navigator to minimize through-plane motion. We assessed the effect of rejected navigators on the relaxation times. Joint T1-T2 maps with four different navigator acceptance window widths (NAWWs from ±4mm to ±32mm) were acquired in 6 healthy volunteers and compared to clinical routine techniques. The accuracy and precision of the maps resulting from the different NAWWs did not significantly differ, suggesting that the NAWW can be chosen as a balance between navigator inefficiency and through-plane motion.
Introduction
Cardiac magnetic resonance fingerprinting (cMRF) relies on varying the magnetization to extract multiple underlying parameters with a single scan. It has previously been applied for breath-hold 2D (1) and free-breathing 3D (2) joint T1 T2 mapping of the myocardium at 1.5T. In case a free-breathing 2D technique is desired, a respiratory navigator can be added to avoid through-plane motion. However, rejected navigators strongly change the magnetization time course and thus the resulting simulated dictionary, which may affect both the accuracy and precision of the technique. The goal of this study was therefore to implement a navigator-gated free-breathing 2D joint T1,T2 cMRF of the myocardium at 3T and to quantify the influence of the navigator acceptance window width (NAWW) on the T1 and T2 accuracy and precision in a phantom and the myocardium of healthy volunteers.Method
A 2D free-breathing EGC-triggered gradient-echo pulse sequence (named PARMA for PARameter MApping) was implemented with single-shot images acquired with a continuous radial golden angle trajectory preceded by 5 different magnetization preparations. These were no preparation, an adiabatic inversion pulse, and 3 different T2 preparation modules (T2 prep=0/25/50ms) to sensitize the contrast to both T1 and T2 relaxation(2,3)[ (Figure 1). This set of five preparations was then repeated five times. Respiratory motion was tracked using a lung-liver navigator before the preparation modules; if the navigator rejected, neither the preparation nor the imaging were activated that heartbeat. The undersampled images were reconstructed using compressed sensing and low rank regularization along the contrast dimension(4). Non-rigid registration was applied to account for residual in-plane motion.A dictionary was simulated for each acquisition using extended phase graphs (EPG (5) ) for a range of T1 (40:10:3000 ms) and T2 (20:1:100; 102:2:230 ms) values in Matlab (The Mathworks) and included slice-profile and inversion-inefficiency corrections (6).
PARMA maps of the ISMRM-NIST phantom (7) were acquired in a 3T clinical scanner (Magnetom Prisma, Siemens Healthcare) with matrix size=192x192, pixel size=(1.56mm)2, slice thickness=8mm, TR=3.49 ms, and TE=1.56ms. Clinical routine T1 and T2 maps (pixel size=(1.8-1.9mm)2, slice thickness 8mm) were acquired using MOLLI (8) and T2-prepared bSSFP T2 mapping (9), respectively. To establish the accuracy of the technique, a linear regression of the resulting relaxation times was made with those of the inversion-recovery and spin-echo gold standard techniques, respectively.
The in-vivo study was approved by the local ethics committee and all participants provided written informed consent. PARMA was applied in N=6 healthy volunteers at a mid-ventricular short-axis orientation with four different NAWW (4mm, 8mm, 16mm, and 32mm) of the lung-liver navigator. MOLLI and T2-prepared bSSFP were obtained during a breath hold.
The total acquisition time was recorded for each PARMA acquisition to quantify the scan time increase caused by the navigator.
The T1 and T2 value of the visible myocardium as well as its intra-patient coefficient of variation (CoV=mean/standard deviation) was determined for each map in each volunteer, and differences were tested with a repeated-measures ANOVA with post-hoc Tukey analysis.
Results
The phantom PARMA T1 and T2 relaxation times agreed well with the gold standard techniques (Figure 2), while the PARMA T1 resulted in a slope closer to identity than MOLLI(1.17 vs 0.76, P<0.001), indicating higher accuracy, although the T1 values within the myocardial range were slightly overestimated (Figure 2 A-B).In the healthy volunteers, the myocardial PARMA T1 values trended to be higher (consistent with the phantom results) but did not significantly differ from MOLLI, except for NAWW=16mm (Figure 3). The T1 CoV, T2 values, and T2 did not differ between the different NAWW acquisitions or the reference techniques. The somewhat higher T1 values and T2 values could also be visibly discerned, as well as a slight reduction in the visible thickness of the myocardium reduced for larger NAWW (Figure 4). As expected, larger NAWW resulted in fewer rejected navigators and thus in shorter acquisition times (Figure 5).
Discussion
PARMA had high accuracy in the phantom and was successfully acquired in all healthy volunteers with all tested NAWW, which also resulted in similar mapped relaxation times and CoV. There was no clear trend for wider or narrower NAWW to lead to higher or lower precision or accuracy. This suggests that a NAWW can be chosen purely as a consideration between increased scan time and acceptable through-plane motion. However, the visible thickness of the myocardium was often slightly reduced with larger NAWW, suggesting that through plane motion may cause partial volume effect with neighbouring tissues, and that large NAWW should be avoided in suspected non-transmural disease.The T1 maps may have a higher CoV than MOLLI due to insufficient T1 weighting, which could be addressed by switching the first two preparation modules or by adding a variable flip angle to the images. The smaller pixel size and regularization weights could also be adapted to compensate. These initial findings should furthermore be confirmed in a larger group of subjects.
In conclusion, we preliminarily demonstrated that navigator rejection in free-breathing cardiac MR fingerprinting at 3T does not affect the accuracy or precision of the mapped relaxation times.
Acknowledgements
This project was funded by a grant from the Swiss National Science Foundation (#CRSII5_202276).References
1. Hamilton JI, Jiang Y, Chen Y, et al. MR fingerprinting for rapid quantification of myocardial T1 , T2 , and proton spin density. Magn. Reson. Med. 2017;77:1446–1458 doi: 10.1002/mrm.26216.
2. Cruz G, Jaubert O, Qi H, et al. 3D free-breathing cardiac magnetic resonance fingerprinting. NMR Biomed. 2020;33:e4370 doi: 10.1002/nbm.4370.
3. Henningsson M. Cartesian dictionary-based native T1 and T2 mapping of the myocardium. Magn. Reson. Med. 2022;87:2347–2362 doi: 10.1002/mrm.29143.
4. Di Sopra L, Roy CW, Bastiaansen JAM, et al. Fully Self-Gated Cardiac and Respiratory Motion-Resolved Isotropic 5D T1 Mapping of the Heart: Preliminary Results. In: Proc Int Soc Magn Reson Med 27. Montréal QC, Canada; 2019.
5. Malik SJ, Padormo F, Price AN, Hajnal JV. Spatially resolved extended phase graphs: modeling and design of multipulse sequences with parallel transmission. Magn. Reson. Med. 2012;68:1481–1494 doi: 10.1002/mrm.24153.
6. Hamilton JI, Jiang Y, Ma D, et al. Investigating and reducing the effects of confounding factors for robust T1 and T2 mapping with cardiac MR fingerprinting. Magn. Reson. Imaging 2018;53:40–51 doi: 10.1016/j.mri.2018.06.018.
7. Stupic KF, Ainslie M, Boss MA, et al. A standard system phantom for magnetic resonance imaging. Magn. Reson. Med. 2021;86:1194–1211 doi: 10.1002/mrm.28779.
8. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn. Reson. Med. 2004;52:141–6 doi: 10.1002/mrm.20110.
9. Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56 doi: 1532-429X-11-56 [pii] 10.1186/1532-429X-11-56.
Figures
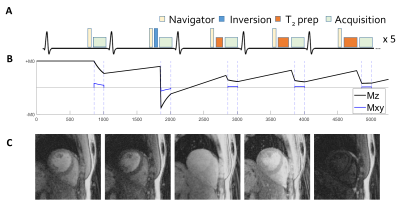
Figure 1: Pulse sequence diagram and example images for the proposed free-breathing radial 2D ECG-triggered MR fingerprinting technique PARMA. A) Pulse sequence diagram. The image are acquired during 25 heartbeats, using inversion pulses and three different T2-prep modules. B) Signal evolution of the myocardium across 5 cardiac cycles. C) Example images of the first 5 cardiac cycles in a healthy volunteer reconstructed using compressed sensing with a low rank regularization in the contrast dimension.
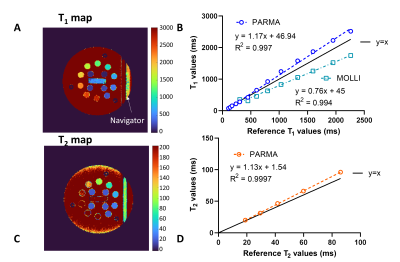
Figure 2: Accuracy of PARMA T1 and T2 maps in the ISMRM-NIST phantom compared to gold-standard techniques. A) T1 map of the reference phantom obtained with PARMA. B) Linear regression plots of T1 values in the phantom in the myocardial T1 mapping range together with the clinical reference MOLLI. C) T2 map of the reference phantom obtained with PARMA. D) Linear regression plots of T1 values in the phantom in the myocardial T2 mapping range. The T2-bSSFP values were not included as the technique did not perform well in this phantom.
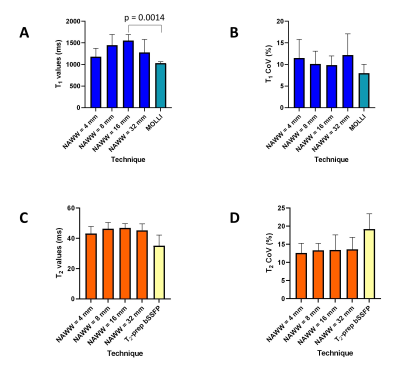
Figure 3: Impact of the navigator acceptance windows width (NAWW) on the values T1 and T2 of the myocardium compared with reference techniques in 6 healthy volunteers. A) Average T1 values of the myocardium for the four NAWW, compared to MOLLI. B) Coefficient of variation (CoV) of the myocardium T1 maps for the four NAWW, compared to MOLLI. C) Average T2 values of the myocardium for the four NAWW, compared to T2-prep bSSFP. D) CoV of the myocardium T2 maps for the four NAWW, compared to T2-prep bSSFP.
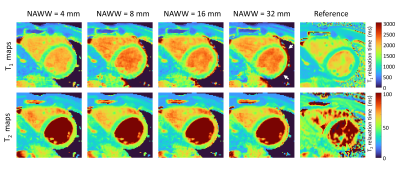
Figure 4: PARMA T1 and T2 maps for the different NAWW compared to the Reference techniques. The NAWW does not significantly change the relaxation times in the resulting PARMA maps. However, the visible thickness of the myocardium is often reduced with larger NAWW (white arrows). The PARMA T1 and T2 values are consistently lower than those obtained with the routine techniques.
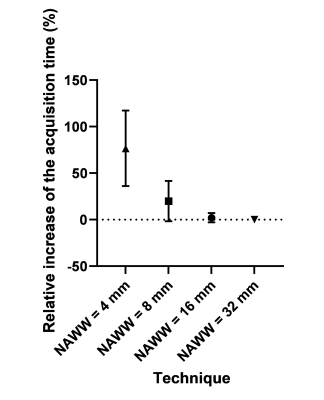
Figure 5: The acquisition time as a function of the NAWW. As expected, the acquisition time decreases when the NAWW increases and a larger part of the navigators are accepted.