3998
Cardiac magnetic resonance in survivors of critical illness: myocardial fibrosis and dysfunction are associated with acute kidney injury1Diagnostic and Interventional Radiology, University Hospital Bonn, Bonn, Germany, 2Clinic for Internal Medicine II, Heart Center, University Hospital Bonn, Bonn, Germany, 3Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany
Synopsis
Keywords: Cardiomyopathy, Quantitative Imaging, T1 and T2 Mapping
In this prospective study, cardiac magnetic resonance (CMR) was performed in 48 intensive care unit (ICU) survivors of acute critical illness without prior known cardiac disease. CMR revealed previously unrecognized functional abnormalities, including reduced left ventricular ejection fraction and impaired strain values, as well as evidence of focal and diffuse myocardial fibrosis represented by positive late gadolinium enhancement lesions and elevated myocardial T1 mapping and ECV. Findings were more pronounced in ICU survivors who had experienced acute kidney injury. Unrecognized fibrotic and functional myocardial alterations may be a correlate of physical impairment in post-intensive care syndrome.
Introduction
Critical illness syndromes are characterized by different acutely life-threatening clinical conditions that usually require intensive care unit (ICU) treatment and, if survived, can lead to physical, cognitive, and psychological impairments1. Several immunometabolic and inflammatory mechanisms promoting cardiovascular disease in response to critical illness have been described, and biomarkers of cardiac failure were associated with reduced long-term survival in patients after ICU treatment2. Acute kidney injury (AKI) is a condition which is one of the most common accompanying complications of critical illness3. AKI is a predictor of short- and long-term adverse cardiovascular events4,5. Reciprocal effects between cardiac and renal disease are referred to as cardiorenal syndrome (CRS), with type 3 CRS describing cardiac disease as a result of AKI6. However, the extent and manner in which critical illness and AKI contribute to myocardial injury requires further investigation.In this prospective, exploratory study, cardiac magnetic resonance (CMR) was performed in ICU survivors of acute critical illness without previously known cardiac disease to investigate the extent of subclinical myocardial abnormalities such as fibrosis, inflammation, or ventricular dysfunction.
Methods
This prospective case-control study was approved by the institutional ethics committee. All participants gave written informed consent. ICU survivors of critical illness were consecutively enrolled from March 2021 to May 2022. Patients with a previous ICU stay (>3 days, between 2015-2021) due to critical illness (defined as an acute life-threatening illness requiring intensive care, e.g., trauma, hemorrhage, stroke, infection, respiratory failure) were retrospectively identified and evaluated for potential study participation. Only participants without previously diagnosed cardiac disease or systemic disease with potential cardiac involvement were included. Post-ICU participants were subdivided into (a) participants who had and recovered from AKI during critical illness (AKI group), and (b) participants without acute or chronic kidney injury during critical illness (non-AKI group). The control group consisted of healthy subjects without previous ICU stay and no cardiac disease history. CMR was performed at 1.5T and the scan protocol allowed for assessment of ventricular volumes, function, mass, and feature-tracking strain (systolic global longitudinal [GLS] and circumferential [GCS]). Myocardial edema was evaluated visually and semi-quantitatively (T2 signal intensity ratio) on T2-weighted images. Late gadolinium enhancement (LGE) was assessed visually and semi-quantitatively (“full width at half maximum” method). Myocardial T1 (based on modified look-locker inversion recovery [MOLLI] scheme) and T2 (based on gradient and spin-echo sequence [GraSE]) mapping, and hematocrit corrected extracellular volume fraction (ECV) were calculated. Student t test, Mann-Whitney U test, χ2 test, Fisher’s exact test, one-way analysis of variance with subsequent Tukey multiple comparison tests, and Kruskal-Wallis test were used for statistical analysis.Results
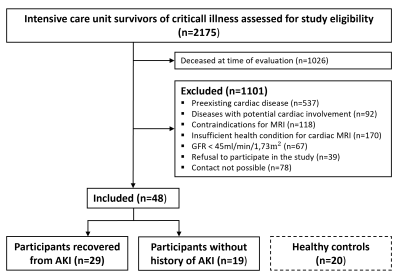
A total of 68 participants were included in this prospective study: 48 ICU survivors of critical illness (mean age ± standard deviation, 46±15 years; 42% women) and 20 healthy controls (48±14 years; 45% women) (Figure 1). The median length of ICU stay was 35 days (IQR, 22-58 days). The median interval between hospital discharge and CMR scan was 27 months (IQR, 13-50 months). 29/48 ICU survivors (60%) had recovered from AKI and 19/48 ICU survivors (40%) had no history of kidney injury.ICU survivors of critical illness had reduced left ventricular ejection fraction (57±6% vs 60±5%; P=0.027) and impaired GLS (‑20.3±3.7% vs ‑23.1±3.5%; P=0.004) and GCS (‑20.3±4.4% vs ‑24.1±2.7%; P=0.001) compared to healthy controls. Subgroup analysis revealed impaired GLS in AKI participants (‑19.9±3.8% vs ‑20.9±3.5%; P=0.008), but not in non-AKI participants compared to the healthy control group. Neither the post-ICU group nor the control group had signs of myocardial edema (e.g., T2 relaxation times: 55±3ms vs 54±2ms; P=0.075).
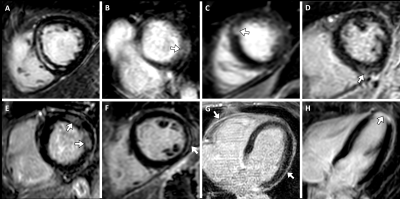
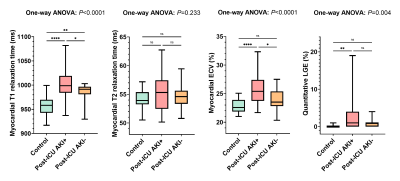
LGE was present in 10/48 ICU survivors (21%; healthy controls: 0/20 [0%]; P=0.027). Of these, 5/10 (50%) had an ischemic pattern, 4/10 (40%) had a non-ischemic pattern, and 1/10 (10%) showed pericardial enhancement (Figure 2). LGE lesions were more frequently observed in the AKI group than in the healthy control group (8/29 [28%] vs 0/20 [0%]; P=0.01) and the non-AKI group (8/29 [28%] vs 2/19 [11%]; P=0.155). Participants with AKI had a higher LGE extent compared with healthy controls (LGE percentage, 1% [IQR, 0-4] vs 0% [IQR, 0-0]; P<0.001) (Figure 3).
Myocardial T1 relaxation times (995±31ms vs 957±21ms; P<0.001) and ECV values (24.9±2.5% vs 22.8±1.2%; P<0.001) were significantly higher in ICU survivors compared to controls. Moreover, the AKI group had higher T1 relaxation times (1002±33ms) and ECV values (25.6±2.6%) compared to the non-AKI group (T1: 983±21ms, P=0.046; ECV: 23.9±1.9%; P=0.017) (Figure 3).
Conclusion
The main findings of our study are (i) that survivors of critical illness had distinct functional and structural cardiac abnormalities including LGE lesions as a marker of focal fibrosis and elevated myocardial T1 relaxation times and ECV as markers of diffuse myocardial fibrosis, and (ii) that these myocardial abnormalities were more abnormal when critical illness was accompanied by severe AKI (type 3 CRS). Our results suggest that survival of critical illness is associated with cardiac sequelae even in patients with undiagnosed heart disease and appears to be exacerbated by severe AKI. Unrecognized fibrotic and functional myocardial changes in survivors of critical illness may be a correlate of physical impairment in post-intensive care syndrome.Acknowledgements
N/AReferences
1. Adhikari NKJ, Fowler RA, Bhagwanjee S et al. Critical care and the global burden of critical illness in adults. Lancet 2010;376:1339–1346.
2. Gayat E, Cariou A, Deye N et al. Determinants of long-term outcome in ICU survivors: results from the FROG-ICU study. Crit Care 2018;22.
3. Pickkers P, Ostermann M, Joannidis M et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med 2017;43:1198–1209.
4. Odutayo A, Wong CX, Farkouh M et al. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J Am Soc Nephrol 2017;28:377–387.
5. Legrand M, Rossignol P. Cardiovascular Consequences of Acute Kidney Injury. N Engl J Med 2020;382:2238–2247.
6. Ronco C, Haapio M, House AA et al. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539.
Figures