3983
Convex Optimized Diffusion Encoding (CODE) with M1-Nulling for Pancreatic Diffusion Weighted Imaging1Radiology, Stanford University, Palo Alto, CA, United States
Synopsis
Keywords: Pancreas, Diffusion/other diffusion imaging techniques, Diffusion Weighted Imaging, M1-nulled, ADC
Multi-shot DWI reduces image distortion while increasing SNR. More recently, motion-compensated diffusion encoding has been shown to reduce artifacts and improve ADC consistency in single-shot DWI. Motion-compensated multi-shot pancreatic DWI may also mitigate artifacts arising from shot-to-shot incoherence. Herein, we conducted a pilot study of multi-shot DWI of the pancreas with and without motion-compensated diffusion encoding. Image quality and artifacts were scored by expert radiologists. ADC values of different pancreatic anatomical segments were computed. Multi-shot DWI with motion-compensated gradient encoding increased homogeneity of signal intensity and ADC values across the pancreas.INTRODUCTION
Pancreatic DWI is clinically useful for pancreatic cancer detection1. In particular, ADC quantification is useful in characterizing pancreatic lesions and assessing treatment response of pancreatic cancer2-5. However, conventional pancreatic DWI is acquired using single-shot spin-echo echo-planar imaging (SE-EPI), which provides challenges associated with image distortion, low signal-to-noise ratio (SNR), and bulk-motion induced image artifacts6. These challenges need to be addressed for pancreatic DWI to emerge as the clinical standard for pancreatic cancer detection. Multi-shot DWI has been shown to reduce image distortion while increasing SNR but can introduce additional image artifacts arising from shot-to-shot phase incoherence7-9. Motion-compensated diffusion encoding can reduce artifacts and improve the consistency of ADC quantification in single-shot DWI sequence6,8. Motion-compensated multi-shot pancreatic DWI could mitigate artifacts arising from shot-to-shot incoherence, but has yet to be investigated. The objective of this study was to evaluate the effect of multi-shot, motion-compensated diffusion encoding on image quality, artifacts, and ADC quantification for pancreatic DWI.METHODS
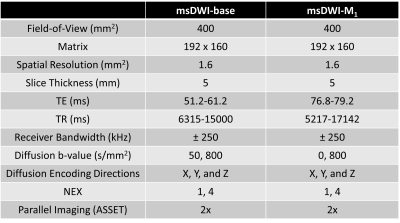
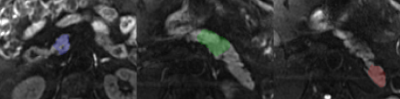
In this prospectively acquired, retrospectively reviewed, pilot study, patients (N=19) underwent abdominal MRI exams with IRB approval and informed consent. In each patient, two DWI sequences were acquired: 1) a vendor-provided SE-EPI multi-shot DWI sequence (msDWI), and 2) a custom multi-shot DWI sequence designed with a real-time convex optimized diffusion encoding (CODE) framework that provided M1-nulled diffusion encoding (msDWI-M1) 8-9. Imaging parameters are summarized in Table 1. All images were acquired using respiratory triggering on a 3T SIGNA scanner (Premier, GE HealthCare, Waukesha, WI) with a 30-channel phased array coil and processed using the vendor-provided reconstruction pipeline. To assess the effect of M1-compensated diffusion encoding on image quality and artifacts, we conducted an observer study performed by two radiologists (R1 and R2), each with fellowship training in abdominal MRI and at least 7 years of experience. Both radiologists were blinded to the clinical data and the DWI sequences. Analysis included blinded pair-wise comparisons of (i) pancreatic boundary delineation; (ii) perceived motion artifact in the pancreas; (iii) signal homogeneity in the pancreatic parenchyma; (iv) perceived noise in the pancreas; and (v) preference for reviewing the pancreas between msDWI and msDWI-M1.(options: left images are better; images are equal; right images are better). All reviews were conducted on the high b-value (800s/mm2) diffusion images. To assess the effect of M1-compensated diffusion encoding on ADC quantification, ADC maps were computed for each of the DWI sequences. A radiologist manually segmented the pancreatic head, body, and tail (Figure 2) on each ADC map. The mean ADC values were computed within the pancreatic head, body, and tail for each sequence. Repeated ANOVA was used to analyze the ADC difference between the three pancreatic segments. If the ANOVA test showed statistically significant differences in ADC, subsequent post-hoc pairwise t-test with Bonferroni correction was used to test which pair of pancreatic segments had different ADC values.RESULTS
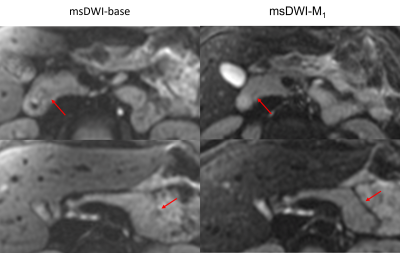
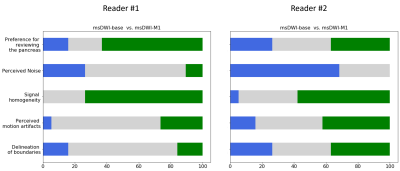
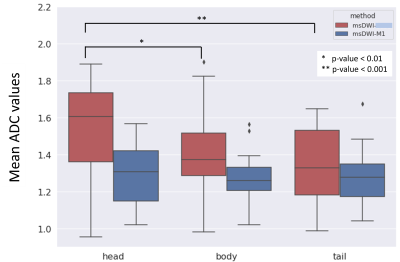
Superior signal homogeneity of the pancreatic parenchyma was observed on msDWI-M1 versus msDWI in over half of cases for both readers (75% and 58% for R1 and R2, Figure 3). Similarly, msDWI-M1 more commonly had reduced perceived motion artifact relative to msDWI artifact (26/42%; R1/R2). msDWI more frequently to had less perceived noise in the pancreatic bed (26/68%; R1/R2). There was little difference in delineation of pancreatic boundaries between the two sets of images for both readers. Overall, both readers preferred msDWI-M1 over msDWI for visualizing the pancreas (63/36%; R1/R2).Mean ADC (um2/us) in the pancreatic head, body, and tail were 1.53±0.26, 1.40±0.22, and 1.35±0.22 for msDWI and 1.30±0.16, 1.27±0.13, 1.27±0.15 for msDWI-M1 (Figure 4). For msDWI, ADC were significantly different between the pancreatic head, body, and tail (p=6e-5). A post-hoc pairwise t-test showed that ADC values were significantly different between the pancreatic head and body (p=0.005), head and tail (p=6e-5), but not the body and tail (p=0.13). For msDWI-M1, ADC values were not significantly different between the pancreatic head, body, and tail (p=0.5). Furthermore, the standard deviation of ADC values in the head, body, and tail of the pancreas were reduced for msDWI-M1 compared to msDWI.DISCUSSION
Diffusion weighted imaging with M1-compensated diffusion encoding increased signal homogeneity and reduced motion-induced artifacts in the pancreas but increased perceived noise due to the prolonged echo times. Pancreatic ADC values found in this study were consistent with prior studies10. Heterogeneity of pancreatic ADC across different anatomical segments were observed in msDWI, which is consistent with prior studies using single-shot DWI11. Recent studies suggested this heterogeneity may be due to motion-induced artifacts and that M1-compensated could potentially eliminate this heterogeneity in single-shot DWI6. ADC values from msDWI-M1 were lower than those from msDWI, possibly reflecting correction of underlying motion-induced signal loss on high b-value images. Similar to these findings, we have shown that in multi-shot DWI, ADC values were not significantly different across the pancreas when M1-compensated diffusion encoding was used.CONCLUSION
Multi-shot DWI with M1-compensated diffusion encoding provides superior homogeneity of both signal intensity and ADC values across the pancreas, thereby improving standardization of pancreatic tissue characterization independent of anatomical location. This technique can potentially reduce bias and improve precision of quantitative DWI of the pancreas.Acknowledgements
This project was supported, in part, by support from GE Healthcare.References
1. Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, Fujii H. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007 Feb;188(2):409-14. doi: 10.2214/AJR.05.1918. PMID: 17242249.
2. Lee SS, Byun JH, Park BJ, Park SH, Kim N, Park B, Kim JK, Lee MG. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28(4):928–936.
3. Zhu M, Zhang C, Yan J, Sun J, Zhao X, Zhang L, Yin L. Accuracy of quantitative diffusion-weighted imaging for differentiating benign and malignant pancreatic lesions: a systematic review and meta-analysis. European Radiology. 2021 Apr 13:1-4. 5.
4. Ren H, Mori N, Hamada S, Takasawa C, Mugikura S, Masamune A, Takase K. Effective apparent diffusion coefficient parameters for differentiation between mass-forming autoimmune pancreatitis and pancreatic ductal adenocarcinoma. Abdominal Radiology. 2021 Apr;46(4):1640-7.
5. Trajkovic-Arsic, M., Heid, I., Steiger, K. et al. Apparent Diffusion Coefficient (ADC) predicts therapy response in pancreatic ductal adenocarcinoma. Sci Rep 7, 17038 (2017).
6. Geng R, Zhang Y, Starekova J, Rutkowski DR, Estkowski L, Roldán-Alzate A, Hernando D. Characterization and correction of cardiovascular motion artifacts in diffusion-weighted imaging of the pancreas. Magn Reson Med. 2021 Oct;86(4):1956-1969. doi: 10.1002/mrm.28846. Epub 2021 Jun 17. PMID: 34142375; PMCID: PMC8295219.
7. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013;72:41-7. 8. Loecher M, Middione MJ, Ennis DB. A gradient optimization toolbox for general purpose time‐optimal MRI gradient waveform design. Magn Reson Med 2020;84(6):3234-45.
9. Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn Reson Med. 2017 Feb;77(2):717-729. doi: 10.1002/mrm.26166. Epub 2016 Feb 22. PMID: 26900872.
10. Schoennagel BP, Habermann CR, Roesch M, Hahne JD, Arndt C, Kleibeler L, Petersen KU, Graessner J, Adam G, Herrmann J. Diffusion-weighted imaging of the healthy pancreas: apparent diffusion coefficient values of the normal head, body, and tail calculated from different sets of b-values. J Magn Reson Imaging. 2011 Oct;34(4):861-5. doi: 10.1002/jmri.22743. Epub 2011 Aug
11. PMID: 21837782. 11. Herrmann J, Schoennagel BP, Roesch M, Busch JD, Derlin T, Doh LK, Petersen KU, Graessner J, Adam G, Habermann CR. Diffusion-weighted imaging of the healthy pancreas: ADC values are age and gender dependent. J Magn Reson Imaging. 2013 Apr;37(4):886-91. doi: 10.1002/jmri.23871. Epub 2012 Oct 19. PMID: 23086728.
Figures