3981
Role of tomoelastography in evaluation for pancreatic fistula after pancreaticoenteric anastomosis1The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
Synopsis
Keywords: Pancreas, Surgery, Magnetic resonance elastographies, Pancreatectomy, Pancreatic fistula
This prospective study enrolled the patients who underwent both preoperative tomoelastography and pancreaticoenteric anastomosis. Eighty-two patients were included (median age: 59.5 years, 40 men, 18 patients with POPF). Main pancreatic duct diameter (MPDD) (P=0.012), c (P<0.001) and φ (P=0.001) were relevant factors for POPF. The area under the curve (AUC) of c, φ and MPDD for predicting POPF was 0.880, 0.816 and 0.747. Fibrosis was the relevant factor of POPF (P=0.001). There was positive correlation between fibrosis and stiffness (r=0.681, P<0.001). Tomoelastography is a novel and robust multi-frequency MRE technique that can facilitate the prediction of POPF.Manuscript
IntroductionPostoperative pancreatic fistula (POPF) is the most common and serious complication of pancreatectomy (1). Preoperative risk stratification of POPF in patients undergoing pancreatectomy facilitates perioperative precision medicine (2, 3). The fistula risk score, obtained perioperatively, from a more practical point of view, tend to be fraught with potential dangers.
Previous studies had been conducted to evaluate the relationship between parameters derived from imaging and pancreatic texture for predicting POPF, such as the signal intensity ratio of T1-weighted MRI (4), magnetization transfer imaging (5) and dual-energy CT (6). Whereas, those quantitative tools cannot directly quantify pancreatic texture but also influenced by other factors. Pancreatic US elastography could be used to assess pancreatic stiffness but suffered from limited reproducibility (6).
Tomoelastography, a novel multi-frequency MRE technique with noise-robust data postprocessing, can generate quantitative maps for biomechanical properties with high-resolution anatomical details (7, 8). To the best of our knowledge, the possibility of using tomoelastography to predict the occurrence of POPF has not been explored.
Therefore, the present study was to prospectively investigate the utility of tomoelastography for preoperative risk prediction for occurrence of POPF in patients undergoing pancreaticoenteric anastomosis.
Materials and Methods
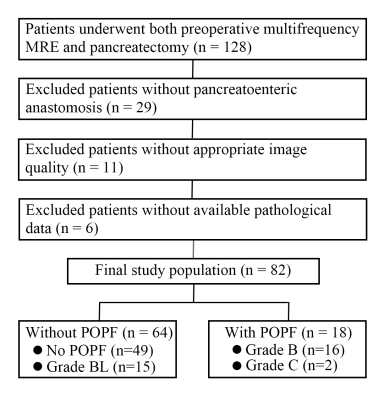
This prospective single-center study was approved by the institutional review board of our hospital and the written informed consent were received from patients. The flow chart was shown in Figure 1.
The grading criteria of pancreatic fibrosis at the resection margin were performed as previously described as 4 grades (9). The exocrine gland atrophy was classified according to the percentage of viable exocrine gland as previously described as 3 grades (10). The grading criteria of lipomatosis were adapted as previously described as 4 grades (10, 11).
The entire pancreatic tomoelastography was scanned in 7 minutes and 22 seconds covered by 35 contiguous axial sections. The multifrequency wave field data was processed at https://bioqic-apps.com.
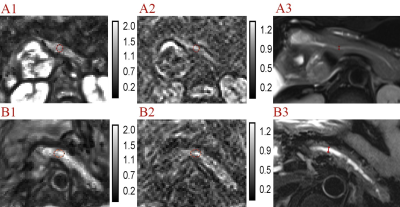
The region of interests (ROIs) (larger than 100 mm3) were placed at the resection margin of pancreas, avoiding visible pancreatic duct dilation, boundary and artifacts, to measure fat fraction (on multi-echo Dixon image), width (on axial fs-T2WI), thickness (on coronal T2WI), stump area (width multiple thickness), stiffness (on c map) and fluidity (on φ map). The main pancreatic duct diameter (MPDD) was also measured (Figure 2).
Results
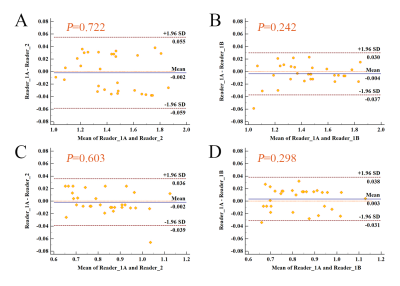
A total of 82 patients were enrolled in this study (median age, 59.5 years, range from 20 to 83 years) and 40 patients were men. There were 18 patients with POPF (ISGPS B and C) among all. The Bland-Altman analysis showed agreements in different readers, different measurements with the same reader (Figure 3). The ICC showed near perfect agreements in c (0.987, P<0.001) and φ (0.993, P<0.001).
There was significant difference between patients with and without POPF in fibrosis (P=0.002), acinar atrophy (P=0.024), MPDD (P=0.001), c (P<0.001) and φ (P<0.001). According to the univariate analysis, fibrosis (P=0.001; OR, 0.29), acinar atrophy (P=0.011; OR, 0.19), MPDD (P=0.012; OR, 0.59), c (P<0.001; OR<0.001) and φ (P=0.001; OR<0.001) were relevant factors of POPF. The only independent relevant factor for preoperatively predicting POPF was c (P<0.001) at the multivariate regression.
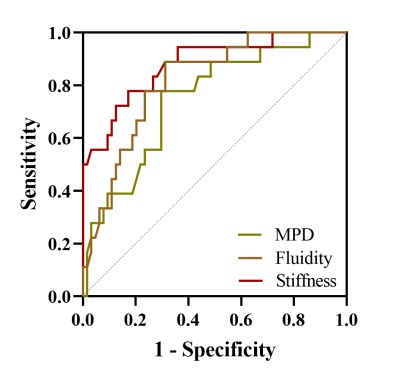
The use of c enabled prediction of POPF with an AUC of 0.880 (cutoff, 1.413 m/sec), which was comparable to the predictive performance of φ (AUC, 0.816; cutoff, 0.796 radian) (NRI: 0.029, P=0.843; IDI: 0.066, P=0.342) and higher than the predictive performance of MPDD (AUC, 0.747; cutoff, 2.67 mm) (NRI: 0.125, P=0.344; IDI: 0.132, P=0.021), as the Figure 4 shown.
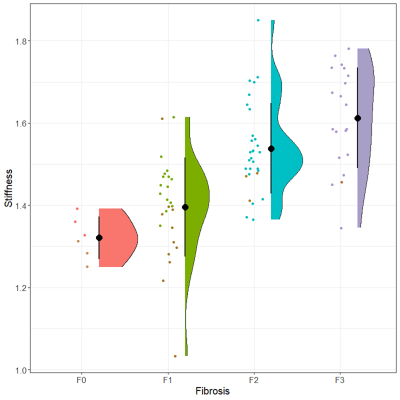
There were 6 patients in F0 fibrosis (3 were with POPF), 26 in F1 (11 were with POPF), 30 in F2 (3 were with POPF), 20 in F3 (1 were with POPF), as the Figure 5 shown. The mean c was 1.321 m/sec in F0 fibrosis, while 1.396, 1.538, 1.612 in F1-3 fibrosis respectively (P<0.001). There was positive correlation between c and fibrosis grades (r=0.681, P<0.001).
Discussion
From the perspective of precision medicine, there is still a clinical need for straightforward and non-invasive modality to quantify pancreatic texture thus preoperatively identify patients at high risk of POPF. In the present study, both mechanical (pancreatic stiffness and fluidity quantified with tomoelastography) and morphologic (MPDD) features were relevant factors for POPF. The predictive performance of pancreatic stiffness was comparable to the fluidity and better than MPDD. Pancreatic fibrosis was the relevant factors for POPF and there was positive correlation between fibrosis and pancreatic stiffness. To the best of our knowledge, this is the first attempt to explore the potential of tomoelastography for preoperative risk prediction for POPF in patients undergoing pancreaticoenteric anastomosis.
In conclusion, tomoelastography is a novel and robust multi-frequency MRE technique that can facilitate the assessment of pancreatic texture. The elevated pancreatic stiffness and fluidity at the resection margin, as quantified by tomoelastography, are mechanical signatures of POPF. Preoperative identification of patients undergoing pancreaticoenteric anastomosis with high-risk POPF using tomoelastography will help improve perioperative precision treatment.
Acknowledgements
The authors sincerely acknowledge Ms. Jing Guo from Department of Radiology of Charité –Universitätsmedizin Berlin, Germany and Mengzhu Wang from MR Scientific Marketing, Siemens Healthineers Ltd. Guangzhou, China for the MR technical support.
References
1. Marchegiani G, Bassi C. Prevention, prediction, and mitigation of postoperative pancreatic fistula. Br J Surg 2021;108(6):602-604. doi: 10.1093/bjs/znab125
2. Trudeau MT, Casciani F, Ecker BL, Maggino L, Seykora TF, Puri P, McMillan MT, Miller B, Pratt WB, Asbun HJ, Ball CG, Bassi C, Behrman SW, Berger AC, Bloomston MP, Callery MP, Castillo CF, Christein JD, Dillhoff ME, Dickson EJ, Dixon E, Fisher WE, House MG, Hughes SJ, Kent TS, Malleo G, Salem RR, Wolfgang CL, Zureikat AH, Vollmer CM, on the behalf of the Pancreas Fistula Study G. The Fistula Risk Score Catalog: Toward Precision Medicine for Pancreatic Fistula After Pancreatoduodenectomy. Ann Surg 2022;275(2):e463-e472. doi: 10.1097/SLA.0000000000004068
3. Smits FJ, van Santvoort HC, Besselink MG, Batenburg MCT, Slooff RAE, Boerma D, Busch OR, Coene P, van Dam RM, van Dijk DPJ, van Eijck CHJ, Festen S, van der Harst E, de Hingh I, de Jong KP, Tol J, Borel Rinkes IHM, Molenaar IQ, Dutch Pancreatic Cancer G. Management of Severe Pancreatic Fistula After Pancreatoduodenectomy. JAMA Surg 2017;152(6):540-548. doi: 10.1001/jamasurg.2016.5708
4. Watanabe H, Kanematsu M, Tanaka K, Osada S, Tomita H, Hara A, Goshima S, Kondo H, Kawada H, Noda Y, Tanahashi Y, Kawai N, Yoshida K, Moriyama N. Fibrosis and postoperative fistula of the pancreas: correlation with MR imaging findings--preliminary results. Radiology 2014;270(3):791-799. doi: 10.1148/radiol.13131194
5. Schawkat K, Eshmuminov D, Lenggenhager D, Endhardt K, Vrugt B, Boss A, Petrowsky H, Clavien PA, Reiner CS. Preoperative Evaluation of Pancreatic Fibrosis and Lipomatosis: Correlation of Magnetic Resonance Findings With Histology Using Magnetization Transfer Imaging and Multigradient Echo Magnetic Resonance Imaging. Invest Radiol 2018;53(12):720-727. doi: 10.1097/RLI.0000000000000496
6. Shi HY, Lu ZP, Li MN, Ge YQ, Jiang KR, Xu Q. Dual-Energy CT Iodine Concentration to Evaluate Postoperative Pancreatic Fistula after Pancreatoduodenectomy. Radiology 2022;304(1):65-72. doi: 10.1148/radiol.212173
7. Garcia SMR, Zhu L, Gultekin E, Schmuck R, Burkhardt C, Bahra M, Geisel D, Shahryari M, Braun J, Hamm B, Jin ZY, Sack I, Guo J. Tomoelastography for Measurement of Tumor Volume Related to Tissue Stiffness in Pancreatic Ductal Adenocarcinomas. Investigative Radiology 2020;55(12):769-774. doi: 10.1097/Rli.0000000000000704
8. Hirsch S, Guo J, Reiter R, Papazoglou S, Kroencke T, Braun J, Sack I. MR Elastography of the Liver and the Spleen Using a Piezoelectric Driver, Single-Shot Wave-Field Acquisition, and Multifrequency Dual Parameter Reconstruction. Magnetic Resonance in Medicine 2014;71(1):267-277.
9. Wellner UF, Kayser G, Lapshyn H, Sick O, Makowiec F, Hoppner J, Hopt UT, Keck T. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB (Oxford) 2010;12(10):696-702. doi: 10.1111/j.1477-2574.2010.00239.x
10. Shi Y, Liu Y, Gao F, Liu Y, Tao S, Li Y, Glaser KJ, Ehman RL, Guo Q. Pancreatic Stiffness Quantified with MR Elastography: Relationship to Postoperative Pancreatic Fistula after Pancreaticoenteric Anastomosis. Radiology 2018;288(2):476-484. doi: 10.1148/radiol.2018170450
11. Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Levy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 2010;148(1):15-23. doi: 10.1016/j.surg.2009.12.005
Figures