3980
Impact of genotype on pancreatic iron overload and impaired glucose metabolism in thalassemia major.1Fondazione G. Monasterio CNR-Regione Toscana, Pisa, Italy, 2Azienda Ospedaliero-Universitaria Ospedali Riuniti "Umberto I-Lancisi-Salesi", Ancona, Italy, 3Gemelli Molise SpA, Fondazione di Ricerca e Cura "Giovanni Paolo II", Campobasso, Italy, 4Azienda Ospedaliera di Rilievo Nazionale Antonio Cardarelli, Napoli, Italy, 5Ospedale “SS. Annunziata” ASL Taranto, Taranto, Italy, 6"ARNAS" Civico, Di Cristina Benfratelli, Palermo, Italy, 7Presidio Ospedaliero Locri - A.S.P di Reggio Calabria, Locri (RC), Italy, 8Ospedale S. Maria Annunziata, Bagno a Ripoli (FI), Italy, 9Ospedale “Di Venere”, Bari, Italy, 10Ospedale “G. Da Saliceto”, Piacenza, Italy, 11Azienda Ospedaliero-Universitaria di Sassari, Sassari, Italy
Synopsis
Keywords: Pancreas, Tissue Characterization
On the basis of the type of gene mutation, three groups of patients with thalassemia major were identified: homozygotes β+, compound heterozygotes β+/β° and homozygotes β°. β0β0 patients were more likely to have pancreatic iron overload than both β+β+ patients and β0β+patients and had a double risk of alterations of glucose metabolism compared to β+β+ patients. Our findings support the knowledge of the different genotypic groups in the clinical management of β-TM patients.Introduction
β-thalassemia major (TM) is characterized by a wide spectrum of clinical manifestations and laboratory findings and the disease phenotype largely depends on the underlying mutations of the β gene. These mutations cause a reduced (β+) or absent (β0) production of the β-globin chain1. No study has evaluated the link between genotype and pancreatic iron, representing a powerful predictor for glucose metabolism and cardiac iron and complications.The objective of this multicenter study was to evaluate the impact of genotype on pancreatic iron levels and glucose metabolism in TM.
Methods
We considered 549 TM patients (36.23±10.63 years, 46.3% females), consecutively enrolled in the Extension-Myocardial Iron Overload in Thalassemia Network.T2* measurements were performed over pancreatic head, body and tail and global value was the mean2,3. The pattern of disturbances of glucose metabolism was assessed by means of the oral glucose tolerance test (OGTT)4.
Results
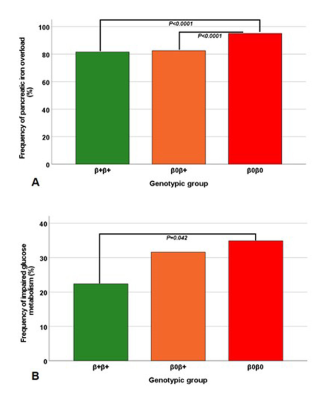
Three groups of patients were identified: homozygous β+ (N=158), compound heterozygous β0β+ (N=206), and homozygous β0 (N=145). The three groups were homogeneous for age, sex, age at start of regular transfusions and chelation, and frequency of splenectomy. The homozygous β0 group showed significantly lower global pancreas T2* values than the homozygous β+ group (9.91±8.13 ms vs 14.45±10.72 ms; P<0.0001) and the heterozygous β0β+ group (9.91±8.13 ms vs 14.69±10.76 ms; P<0.0001). Pancreatic iron overload (T2*<26 ms2) was found in 81.6% of β+β+ patients, 82.5% of β0β+ patients, and 95.1% of β0β0 patients (P<0.0001) (Figure 1A). β0β0 patients were more likely to have pancreatic iron overload than both β+β+ patients (odds ratio-OR=4.39, 95%CI=2.01-9.61; P<0.0001 ) and β0β+patients (OR=4.14, 95%CI=1.94-8.86; P<0.0001). An altered OGTT was found in 154 patients (34 impaired fasting glucose, 54 impaired glucose tolerance, and 66 diabetes mellitus), all showing pancreatic iron overload. The prevalence of impaired OGTT was significantly higher in the homozygous β0 group than in the homozygous β+ group (34.9 vs 22.4%; P=0.042) (Figure 1B). β0β0 patients had around twice the risk of alterations of glucose metabolism (OR=1.86, 95%CI=1.33-3.07; P=0.014) than β+β+ patients.Conclusions
In TM the homozygous β0 genotype is associated with higher pancreatic iron levels and prevalence of impaired glucose metabolism. Therefore, the knowledge of the genotype can be useful in the prediction of some phenotypic features and in the clinical and instrumental management of TM patients.Acknowledgements
We would like to thank all the colleagues involved in the E-MIOT project (https://emiot.ftgm.it/). We thank all patients for their cooperation.References
1. Thein SL. The molecular basis of beta-thalassemia. Cold Spring Harb Perspect Med. 2013;3(5):a011700.
2. Restaino G, Meloni A, Positano V, et al. Regional and global pancreatic T*(2) MRI for iron overload assessment in a large cohort of healthy subjects: Normal values and correlation with age and gender. Magn Reson Med. 2011;65(3):764-769.
3. Meloni A, De Marchi D, Positano V, et al. Accurate estimate of pancreatic T2* values: how to deal with fat infiltration. Abdom Imaging. 2015;40(8):3129-3136.
4. De Sanctis V, Soliman AT, Elsedfy H, et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr J Hematol Infect Dis. 2016;8(1):e2016058.