3972
New Rapid 3D diffusion weighted imaging using 3D hybrid Radial-Echo Planar Imaging (RAZER) sequence1UCAIR, Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
Synopsis
Keywords: Pulse Sequence Design, Diffusion/other diffusion imaging techniques, non-cartesian trajectory
Non-Cartesian sampling schemes offer advantages over Cartesian schemes. Recently 2D radial and 3D hybrid radial-Cartesian approaches such as 3D stack of stars have gained popularity. Despite of many advantages, the current hybrid trajectory is presently limited by drawbacks including the degradation of sampling efficiency and the k-space coverage per unit time for diffusion application. For more flexible, rapid, and motion-robust 3D DWI, we implemented a 3D hybrid radial-EPI(RZAER) sequence and demonstrated the feasibility of 3D diffusion weighted RAZER sequence for carotid vessel wall.
PURPOSE
While Cartesian sampling is widely used in conventional MRI, non-Cartesian sampling schemes such as radial1 or spiral sampling2 offer advantages over Cartesian schemes. A key advantage of schemes that oversample the center of k-space is the intrinsic insensitivity to motion, and the ability to generate images with high spatio-temporal resolution from limited data.3 4 Recently 2D radial and 3D hybrid radial-Cartesian approaches such as 3D stack of stars have gained popularity because they are relatively straight forward and have well understood image reconstruction and artifacts.5,6 Despite of many advantages as mentioned above, the current hybrid trajectory is presently limited by drawbacks including the degradation of sampling efficiency and the k-space coverage per unit time for clinical or other applications.7,8 For more flexible, rapid, and motion-robust 3D sampling, new 3D hybrid trajectory techniques have been introduced.9,10 The incorporation of angular twisting into kx-ky plane to form FID signal of EPI readouts 9 creates an opportunity to reduce acquisition time for improved sampling and SNR efficiency. Multishot diffusion imaging is very sensitive to phase errors due to variable motions during the diffusion gradients. In this work, we implemented diffusion preparation encoding in conjunction with a 3D RAZER sequence which is radial in-plane and Cartesian with an echo train in the partition direction. 9,10METHODS
Pulse Sequence Implementation: We incorporated RAZER trajectory into a Siemens product 3D segmented EPI pulse sequence. In contrast to standard multi- shot 3D EPI, for which readout planes are parallel and offset in a second phase-encoding direction (ky), each radial view sampling (kx-ky) is parallel and offset in a kz-direction. The sequence has a flexibility to acquire the whole kz space for each view angle within a single shot or with segmented multi-shots.All MRI studies were performed on a 3T MRI scanner (Prisma, Siemens Healthineers) with 20 channel head coils or 8 channel dedicated carotid coils.12 Human studies were approved by the institutional review board and obtained signed consent form.
Phantom Scan: A cylindrical phantom filled with a uniform solution and containing some plastic structure inserts was scanned with 3D Radial-EPI sequence with following acquisition parameters: TE/TR=7.3/36ms, 64 radial views, ETL=11, and 12 or 24 slices for single or double shots in EPI readouts, scan time=3.8 or 7.2 sec, respectively.
DWI scan: To test the feasibility of 3D DWI of the proposed sampling scheme, our 3D Radial-EPI sequences were applied to the phantom and to the carotids of a healthy subject. For a comparison, a 2D single shot (ss-) EPI sequence was performed on the same phantom and subject. 3D radial-EPI acquisition parameters were TE/TR=8.2/75ms, 220 radial views, and 11 ETL, scan time for two b values (10, 450 sec/mm2) was 55 sec. The imaging parameters of 2D ss-EPI were TE/TR=75/4500 ms, matrix size=160x160, number of slices = 12, scan time with two b values (0, 500 sec/mm2) was 22 sec.
Reconstruction: All reconstructions were performed offline using code written in MATLAB (MathWorks, Natick, MA) on a Linux workstation. The k-space data was gridded using a Kaiser-Bessel kernel and density compensated using a Voroni-based approach. Data from the multiple receive coils were reconstructed using an optimal coil combine utilizing the inverse covariance matrix derived from coil noise.13,14
RESULTS
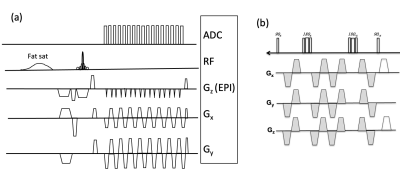
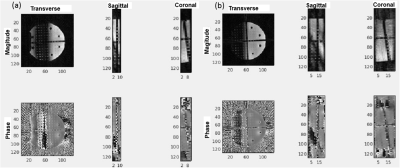
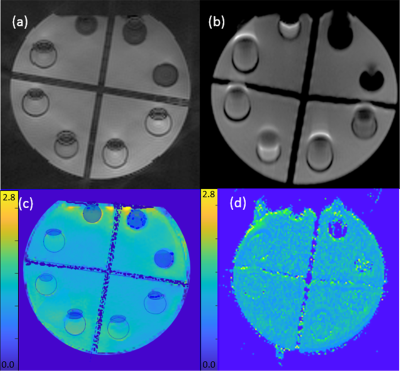
Figure 1 shows the sequence diagram of the RAZER (a) for one radial angle view with 17 echo train and diffusion preparation (b). Figure 2 shows reconstructed phantom images including magnitude and phase images from RAZER acquisitions. Comparing single shot EPI blip acquisition (a), two segmented EPI acquisitions provide more slice coverage with a trade-off of scan time when the same ETL was applied (b). Figure 3 shows the representative diffusion image (b=10 sec/mm2) and ADC map of the phantom. 3D RAZER trajectory provides a clear definition of the phantom internal structure without the geometric distortion artifact which is present in the 2D ss-EPI acquisition. The mean ADC of the solution was 1.21±0.32 and 1.15±0.02 x10-3 mm2/sec from RAZER and 2D ss-EPI acquisitions, respectively. Figure 4 shows a carotid DWI and ADC map at two different slice locations along the carotid arteries obtained from 3D Radial-EPI (top) and 2D-ss EPI (bottom). Despite the higher SNR demonstration of 2D ss-EPI DWI (e, g) and ADC maps(f, h), 3D radial-EPI ADC maps (b, d) provide a better shape of arteries and other anatomy without geometric distortion. The mean ADC of arterial wall was 1.43±0.62 and 1.38±0.18 x10-3mm2/sec from RAZER and 2D ss-EPI acquisition, respectively.DISCUSSIONS
The mean ADC values obtained from RAZER closely approached the values from 2D ss-EPI with higher standard deviation. While the lower SNR of RAZER contributes the higher variation on the ADC, the scan time of two b values within one minute is promising to achieve widespread clinical practice. To improve the SNR and the accuracy of the diffusion measurement, 3D radial-EPI can be performed with signal averaging of multiple measurements without phase error discrepancy induced by motion between different averages. In the future, we will modify this sequence to incorporate a golden angle increment or other view orderings, phase correction of the EPI readouts,11 and gradient delay adjustment8 during radial view sampling to offer improved performance or greater practicality.Acknowledgements
Supported by funds from Huntsman endowed chair, S10 for PrismaReferences
1. Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med. 1992;28(2):275-289. doi:10.1002/MRM.1910280209
2. Liao JR, Pauly JM, Brosnan TJ, Pelc NJ. Reduction of motion artifacts in cine MRI using variable-density spiral trajectories. Magn Reson Med. 1997;37(4):569-575. doi:10.1002/MRM.1910370416
3. Vigen KK, Peters DC, Grist TM, Block WF, Mistretta CA. Undersampled projection-reconstruction imaging for time-resolved contrast-enhanced imaging. Magn Reson Med. 2000;43(2):170-176. doi:10.1002/(SICI)1522-2594(200002)43:2<170::AID-MRM2>3.0.CO;2-P
4. Sarty GE, Bennett R, Cox RW. Direct reconstruction of non-Cartesian k-space data using a nonuniform fast Fourier transform. Magn Reson Med. 2001;45(5):908-915. doi:10.1002/MRM.1120
5. Kim SE, Parker DL, Roberts JA, et al. Differentiation of symptomatic and asymptomatic carotid intraplaque hemorrhage using 3D high-resolution diffusion-weighted stack of stars imaging. NMR Biomed. 2021;34(11). doi:10.1002/NBM.4582
6. Bangiyev L, Raz E, Block TK, et al. Evaluation of the orbit using contrast-enhanced radial 3D fat-suppressed T1 weighted gradient echo (Radial-VIBE) sequence. Br J Radiol. 2015;88(1054). doi:10.1259/BJR.20140863
7. Seo N, Park SJ, Kim B, et al. Feasibility of free-breathing dynamic contrast-enhanced MRI of the abdomen: a comparison between CAIPIRINHA-VIBE, Radial-VIBE with KWIC reconstruction and conventional VIBE. Br J Radiol. 2016;89(1066). doi:10.1259/BJR.20160150
8. Johnson KM. Hybrid radial-cones trajectory for accelerated MRI. Magn Reson Med. 2017;77(3):1068-1081. doi:10.1002/MRM.26188/ASSET/SUPINFO/MRM26188-SUP-0001-SUPPFIGS.PDF
9. Jonathan S v., Vakil P, Jeong YI, Menon RG, Ansari SA, Carroll TJ. RAZER: a pulse sequence for whole-brain bolus tracking at high frame rates. Magn Reson Med. 2014;71(6):2127-2138. doi:10.1002/MRM.24882
10. Jeong HJ, Eddleman CS, Shah S, et al. Accelerating time-resolved MRA with multiecho acquisition. Magn Reson Med. 2010;63(6):1520-1528. doi:10.1002/MRM.22373
11. Graedel NN, McNab JA, Chiew M, Miller KL. Motion correction for functional MRI with three-dimensional hybrid radial-Cartesian EPI. Magn Reson Med. 2017;78(2):527-540. doi:10.1002/MRM.26390
12. Beck MJ, Parker DL, Bolster BD, et al. Interchangeable neck shape–specific coils for a clinically realizable anterior neck phased array system. Magn Reson Med. 2017;78(6):2460-2468. doi:10.1002/MRM.26632
13. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16(2):192-225. doi:10.1002/MRM.1910160203
14. Parker DL, Payne A, Todd N, Hadley JR. Phase reconstruction from multiple coil data using a virtual reference coil. Magn Reson Med. 2014;72(2):563-569. doi:10.1002/MRM.24932
Figures