3961
In Vivo Diffusion MRI at 7 T: High Spatial-Angular-Temporal Resolution Pursuit1Artificial Intelligence in Biomedical Engineering, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Institute of Radiology, University Hospital Erlangen, Erlangen, Germany
Synopsis
Keywords: Image Reconstruction, Diffusion/other diffusion imaging techniques, Ultra high field, multi-shell, q-space, crossing fiber
The pursuit of high-spatial-angular-temporal resolution for in vivo diffusion MRI at 7T is challenging, but also receives continuous interest. We hereby propose shift-encoded interleaved EPI and a joint reconstruction technique with LLR regularization. Preliminary results achieve up to 8.7-fold acceleration per shot in 2-shot EPI acquisition with 1.4 mm isotropic nominal resolution. Moreover, with the integrated joint reconstruction for noise reduction, high-quality diffusion-weighted images render more spatially-continuous fiber anisotropy maps and clearer fiber crossing in the fiber orientation distribution function.Introduction
The pursuit of ultra-high spatial-angular-temporal resolution diffusion MRI at ultra-high field strength (e.g. 7 T) has been important in understanding brain microstructure and function. Such pursuit, however, encounters several technical challenges. First, increased susceptibility and shorter $$$T_2$$$ relaxation at 7 T require faster echo train readouts in echo planar imaging (EPI)1. Second, high angular resolution in $$$q$$$-space requires the use of high or even multiple b-values, e.g. HARDI2, which prolongs the scan time.To address these challenges, we implement a modified interleaved EPI3 sequence, achieving complementary $$$k$$$-$$$q$$$-space sampling. Moreover, we develop a joint reconstruction technique that accomplishes two tasks, (1) shot-to-shot phase variation estimation via joint shot and diffusion encoding reconstruction, and (2) shot-combined diffusion-weighted image update via phase-informed joint diffusion encoding reconstruction.
Here, we present two diffusion acquisition protocols based on 1- and 2-shot EPI, respectively. Single-shot EPI is widely used in clinical diffusion MRI but supplies limited spatial resolution. In contrast, multi-shot EPI can provide higher spatial resolution with shorter echo train length and reduced susceptibility.
Methods
For data acquisition, we employ the interleaved EPI sequence. Its in-plane sampling pattern is modified such as to realize one $$$k_y$$$ line shift per repetition with the cycling period as the undersampling factor per diffusion encoding. This creates complementary $$$k$$$-$$$q$$$-space sampling.In vivo measurements were conducted at 7 T (Terra, Siemens Healthineers, Erlangen, Germany) with single-slice excitation, 1.4 mm isotropic nominal resolution and 68 slices for whole brain coverage.
Three-shell diffusion sampling was used, with 20 directions for b-value 500 s/mm2, 30 directions for b-value 1000 s/mm2, and 64 directions for b-value 2500 s/mm2, respectively. b0 (non-diffusion-weighted) acquisition was interspersed every 10 diffusion encodings, resulting in a total of 126 diffusion sampling.
Three-fold in-plane acceleration and 6/8 partial Fourier were used, yielding 9 minutes and 15 minutes total acquisition time for 1-shot and 2-shot EPI, respectively. For 2-shot EPI, this corresponds to 8.7-fold acceleration per shot.
For image reconstruction, we jointly update all shot images by minimizing the following equation
$$\sum_{j=1}^{N_\text{coil}} \sum_{s=1}^{N_\text{shot}} \sum_{q=1}^{N_\text{diff}}|| y_{j,s,q} - W_{q,s} F \{ c_j \cdot x_{q,s} \} ||_2^2 + \lambda ||x||_*$$ (1)
Here, $$$x_{q,s}$$$ represents the image from the $$$s$$$th shot and $$$q$$$th diffusion encoding. $$$c_j$$$ is the $$$j$$$ th coil sensitivity map estimated by ESPIRiT4, $$$F$$$ is the 2D FFT, and $$$W_{q,s}$$$ is the sampling mask. We employ the locally low-rank (LLR) regularization5,6,7,8, which has been implemented with integrated SigPy9 and PyTorch features. Equation (1) generalizes to both single-shot and multi-shot EPI diffusion-weighted MRI reconstruction. To resemble SNR in multi-shot acquisition, shot-to-shot phase variation ($$$\theta_{q,s} = \angle{x_{q,s}}$$$) is extracted and incorporated into the forward model10,11,12, thus
$$\sum_{j=1}^{N_\text{coil}} \sum_{s=1}^{N_\text{shot}} \sum_{q=1}^{N_\text{diff}}||y_{j,s,q} - W_{q,s} F \{ c_j \cdot \theta_{q,s} \cdot x_{q} \} ||_2^2 + \lambda ||x||_*$$ (2)
The phase variation can be estimated either by parallel imaging or by our joint reconstruction formulation in (1) from the central k-space data. Minimizing Equation (2) supplies shot-combined diffusion-weighted images. The reconstructed diffusion-weighted images were fed into DIPY13 for the fitting of color-coded fiber anisotropy (FA) and fiber orientation distribution function (fODF)14 maps.
Results
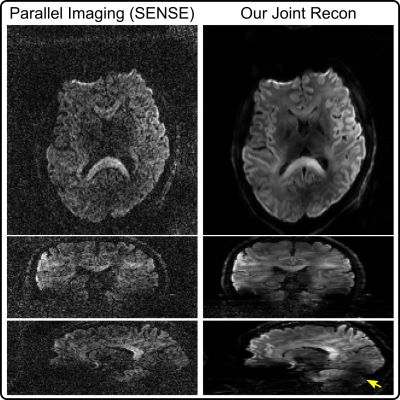
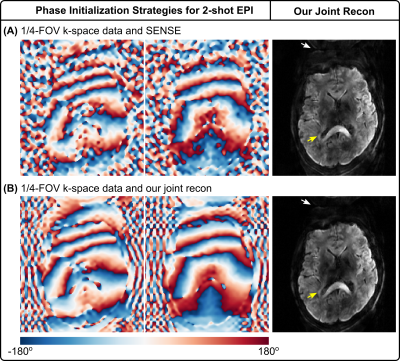
As shown in Figure 1, diffusion-weighted images based on single-shot EPI and SENSE15 at the high b-value 2500 s/mm2 suffer from severe noise. This problem is alleviated using our joint reconstruction with LLR. With single-channel RF excitation, residual B1 inhomogeneity is visible in the sagittal view (yellow arrow).Figure 2 investigates phase initialization strategies. Reference methods8,10,11,12 employ SENSE to reconstruct shot images, from which phase is extracted and smoothed. These methods, however, suffer from blurring and noisy artifacts in shot-combined diffusion-weighted images at high undersampling, as shown in Figure 2 (A). Figure 2 (B) shows that it is beneficial to use joint reconstruction in (1) for shot-to-shot phase variation initialization, which reduces background noise (indicated by white arrows).
Moreover, compared to the axial diffusion-weighted image in Figure 1, 2-shot EPI is capable of reducing spatial distortion, but also revealing the thin fiber surrounding ventricles (indicated by yellow arrows). This fiber structure is not visible from single-shot EPI, potentially due to spatial blurring and distortion. Note that the image contrast differs between 2-shot and 1-shot EPI, because of different TE used.
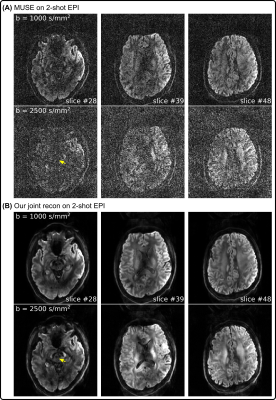
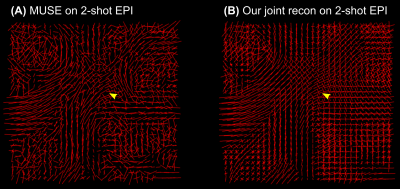
Figure 3 compares 2-shot EPI diffusion-weighted image reconstruction results from MUSE11 and our proposed joint reconstruction, respectively. Here, our joint reconstruction goes beyond one-by-one diffusion-weighted image reconstruction such as MUSE and exploits multi-dimensional low rankness. Note that the red nucleus is only visible from the joint reconstruction, whereas MUSE reconstruction suffers from severe noise at the high b-value (2500 s/mm2).
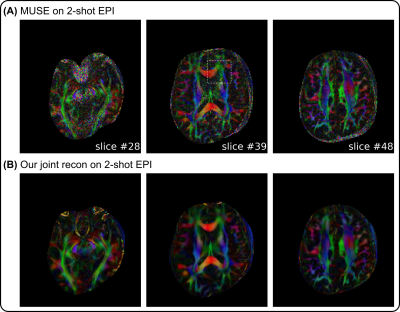
The advantages of joint reconstruction with LLR are especially evident in Figures 4 and 5. First, the FA maps illustrate more spatial continuity compared to MUSE. Second, the fODF map displays clearer fiber crossing within white matter.
Discussion & Conclusion
This work develops shift-encoded interleaved EPI and a joint reconstruction technique with LLR regularization for high-quality diffusion-weighted MRI at 7 T. Preliminary results show that (I) the high spatial-angular-temporal resolution pursuit at 7 T is plausible, (II) minimal geometry distortion and reduced ghosting can be achieved via self-navigated phase variation estimation and joint reconstruction, and (III) integrated reconstruction for noise reduction is advantageous for quantitative diffusion tensor imaging.Acknowledgements
No acknowledgement found.References
[1] Mansfield P. Multi-planar image formation using NMR spin echoes. J Phys C 1977;10:55-58. doi: 10.1088/0022-3719/10/3/004.
[2] Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 2002;48:577-582. doi: 10.1002/mrm.10268.
[3] Butts K, Riederer SJ, Ehman RL, Thompson RM, Jack CR. Interleaved echo planar imaging on a standard MRI system. Magn Reson Med 1993;31:67-72. doi: 10.1002/mrm.1910310111.
[4] Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med 2014;71:990-1001. doi: 10.1002/mrm.24751.
[5] Cai JF, Candes EJ, Shen Z. A singular value thresholding algorithm for matrix completion. SIAM J Optim 2010;20:1956-1982. doi:10.1137/080738970.
[6] Trzasko J, Manduca A. Local versus global low-rank promotion in dynamic MRI series reconstruction. Proc. ISMRM 2011;19:4371.
[7] Zhang T, Pauly JM, Levesque IR. Accelerated parameter mapping with a locally low rank constraint. Magn Reson Med 2015;73:655-661. doi: 10.1002/mrm.25161.
[8] Hu Y, Wang X, Tian Q, Yang G, Daniel B, McNab J, Hargreaves B. Multi-shot diffusion-weighted MRI reconstruction with magnitude-based spatial-angular locally low-rank regularization (SPA-LLR). Magn Reson Med 2020;83:1596-1607. doi: 10.1002/mrm.28025.
[9] Ong F, Lustig M. SigPy: A Python package for high performance iterative reconstruction. Proc. ISMRM 2019;27:4819.
[10] Liu C, Moseley ME, Bammer R. Simultaneous phase correction and SENSE reconstruction for navigated multi-shot DWI with non-Cartesian k-space sampling. Magn Reson Med 2005;54:1412-1422. doi:10.1002/mrm.20706.
[11] Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage 2013;72:41-47. doi:10.1016/j.neuroimage.2013.01.038.
[12] Dai E, Mani M, McNab JA. Multi-band multi-shot diffusion MRI reconstruction with joint usage of structured low-rank constraints and explicit phase mapping. Magn Reson Med 2022. doi: 10.1002/mrm.29422.
[13] Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, Nimmo-Smith I, Dipy Contributors. DIPY, a library for the analysis of diffusion MRI data. Front Neuroinform 2014;8:1-17. doi: 10.3389/fninf.2014.00008.
[14] Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage 2007;35:1459-1472. doi: 10.1016/j.neuroimage.2007.02.016.
[15] Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952-962. doi:10.1002/(SICI)1522-2594(199911)42:5<952::AID-MRM16>3.0.CO;2-S.
Figures