3947
Can NMR phytometabolomics play a role in prevention and management of obesity?1NMR, All India Institute of Medical Sciences, New Delhi, India, 2Department of NMR, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Keywords: Data Acquisition, Spectroscopy, proton NMR spectroscopy, in vitro spectroscopy, obesity
Obesity, a disorder of lipid metabolism, has become a serious health issue globally. Recently, research on pungent phytochemicals and plants with nutritional and medicinal values has gained interest in obesity management. In this study, phytochemicals (n=21) and medicinal plants (n=38) from pungent and non-pungent groups were studied using proton NMR phytometabolomics for their anti-obesity properties. Multivariate analysis of NMR data demonstrated the potential of proton NMR metabolomics in differentiating medicinal plants and their active phytochemicals with anti-obesity properties. These were further confirmed with anti-lipase assays and alkaloid analysis (indicating presence of pungent molecules) of the medicinal plants.
Introduction
As per WHO, obesity is a global epidemic.1 However, unlike other epidemic diseases, obesity is associated with many co-morbidities such as hypertension, diabetes, dyslipidemia, cardiovascular diseases and even some forms of cancer. Medical and surgical interventions are conventional methods of obesity management but at the same time there is also growing attention on the role of diet and nutrition in controlling obesity. In recent times, there has been interest in medicinal and nutraceutical plants in this context. In this study, pungent medicinal plants with potential anti-obesity property have been studied to explore the role of phytometabolomics in prevention and management of obesity.Materials and methods
Phytochemicals: Twenty-one phytochemicals (Merck, USA) from two chemosensory categories were studied: Non-pungent (sweet) (n=14) - sucrose, fructose, dextrose, xylose, glucose, galactose, maltose, mannose, sorbitol, serine, glycine, alanine, histidine, proline; Pungent (n=7) - capsaicin, piperine, allylisothiocyanate, thymol, 6-gingerol, menthol and zingerone.Medicinal plants: Thirty-eight authenticated medicinal and nutraceutical plants from non-pungent (n=17) and pungent (n=21) categories were obtained in dry form: Non-pungent- Vitis vinifera, Phoenix sylvestris, Cocos nucifera root, Musa paradisiaca, Cassia fistula, Cissus quadrangularis, Plantago ovata, Sida cordifolia, Coccinia grandis, Tribulus terristris, Phaseolus trilobus, Cocos nucifera flower, Borassus flabellifer, Prunus amygdalus, Aconitum ferox, Pueraria tuberosa, and Glycyrrhiza glabra; Pungent- Piper longum root, Piper longum fruit, Piper nigrum, Piper chaba, Carum carvi, Brassica juncea, Mentha piperata, Cinnamomum tamala, Zingiber officinale, Anacyclus pyrethrum, Cassia tora, Plumbago zeylanica, Baliospermum montanum, Leucus cephalotus, Croton tiglium, Cuminum cyminum, Gossipium herbaceum, Trigonella foenum-graceum, Erythina indica, Alpinia officinarum and Euphorbia nerifolia.
Sample preparation: The dried plant samples (100gm) were cut to small pieces and soaked in 1000 ml water (mQ) for 24 hours. The method of cold maceration was used to obtain the extracts,2 which were then lyophilised and stored for further studies. For NMR studies, phytochemicals (20μM) and lyophilised powder (25mg/ml) were dissolved in D2O. The phytochemicals which were insoluble in water were dissolved in CDCl3.
NMR studies: 1D proton NMR spectra were acquired at 700 MHz (Agilent, USA) using the following parameters: relaxation delay- 14 sec; spectral width- 12 ppm; scans- 16 (phytochemicals), 64 (plant samples); data points- 32 K (phytochemicals), 64 K (plant samples). Deuterated TSP in a coaxial insert served as an external reference.
Data analysis: NMR spectra were binned and bucketed at intervals of 0.04 ppm (Mnova 10.0, Mestrelab Research) for multivariate analysis. Partial Least Squares Discriminant Analysis (PLS-DA) (Metaboanalyst 5.0) was carried out to study the cluster pattern.
Anti-lipase assay: The enzyme porcine pancreatic lipase (80 μL) was incubated with 10μL plant extract for 30 minutes at 30o C. Para nitro-phenyl butyrate substrate (10μL ) was then added to the incubated enzyme and this mixture was further incubated for 30 mins at 37o C. The end product of enzyme kinetics, p-nitrophenol (which will not form in presence of lipase inhibitor) was observed at 405 nm using Multimode Spectramax UV spectrophotometer.
Quantitative alkaloid analysis: The alkaloid content of the plant extracts were evaluated using standard gravimetric alkaloid tests.3
Results
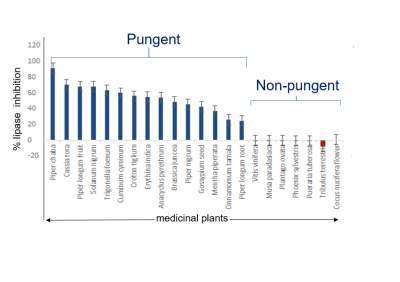
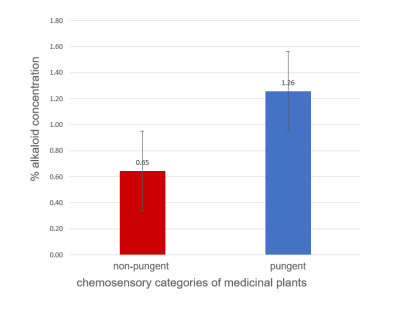
Figure 1 shows representative proton NMR spectra of plants from non-pungent (Musa paradasiaca) and pungent (Piper longum) categories. Spectral differences (shown highlighted) were observed between the two categories in the amino acids (0.5-3.0 ppm) and secondary metabolites (6.0-8.5 ppm) regions. PLS-DA score plots of proton NMR spectral data from phytochemicals (Fig. 2a.) and medicinal plants (Fig.2b.) showed clear discrimination between the pungent and non-pungent plant groups indicating that NMR can differentiate plants based on their pungent property. Similar chemosensory based differences were seen in the results from both anti-lipase assays and alkaloid analysis. Figure 3 shows significant differences (p<0.01) in the % lipase inhibition between the pungent and non-pungent categories of medicinal plants: Pungent (30 to 90% inhibition); Non-pungent (0 to -7 %). Figure 4 shows statistically significant difference (p<0.05) in the presence of alkaloids between the two chemosensory groups. It is pointed out that alkaloids contribute significantly to pungent taste. For example, alkaloids such as capsaicin and piperine are pungent in taste and are also reported to exhibit anti-obesity activity.4,5Conclusion
From being an epidemic, obesity is now transiting to a pandemic, raising serious concerns in healthcare.6 It is now well established that obesity management is not only drug based but also requires diet and lifestyle changes. In this context, there is increasing interest in nutritional therapeutics, prevention and new plant based anti-obesity drug molecules for management of obesity. The current work addresses all these three important requirements of obesity management. For example, the pungent plants with significant anti-lipase activity can be explored further for identifying anti-obesity drug molecules. Some of the studied pungent plants such as Piper longum and Zingiber officinalis are dietary plants and can be used as part of diet for the predisposed risk groups for obesity and can also be explored for anti-obesity nutraceuticals. NMR phytometabolomics can thus be a quick tool for differentiating pungent plants with anti-obesity activity.Acknowledgements
The work was supported by National Medicinal Plant Board, Ministry of Ayush and Science and Engineering Research Board, Government of India.
References
1. “Obesity” WHO. World Health Organization. Web. 2022. (https://www.who.int/health-topics/obesity#tab=tab_1) (accessed through webpage)
2. Wu C, Wang F.,et al. A comparison of volatile fractions obtained from Lonicera macranthoides via different extraction processes: ultrasound, microwave, Soxhlet extraction, hydrodistillation, and cold maceration. Integr. Med. Res. 2015,171-177.
3. British Pharmacopoeia London, Her Majesty’s Stationary Office England, 1980, (I) 316
4. Zheng J, Zheng S.,et al. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci Rep. 2017;37.
5. Ui-Hyun P, Hong-Suk J.,et al. Piperine, a Component of Black Pepper, Inhibits Adipogenesis by Antagonizing PPARγ Activity in 3T3-L1 Cells. J. Agric. Food Chem. 2012, 3853–3860.
6. Meldrum DR, Morris MA, et al. Obesity pandemic: causes, consequences, and solutions-but do we have the will? Fertil Steril. 2017, 833-839.
Figures

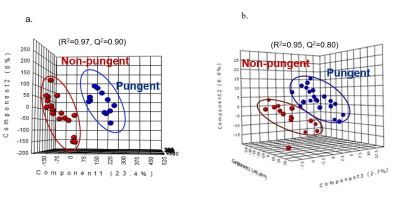
3D plots of Partial Least Square Discriminant Analysis of the NMR spectral data showing discrimination between non-pungent and pungent chemosensory groups of (a) phytochemicals and (b) medicinal plants