3939
31P MRSI coil combination using 23Na sensitivity information acquired with the same loop array at 7T: preliminary verification1UMC Utrecht, Utrecht, Netherlands, 2Tesla Dynamic Coils B.V., Zaltbommel, Netherlands, 3Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
Keywords: Image Reconstruction, Spectroscopy
We utilized a quintuple-tuned RF head coil array by using the high-SNR 23Na signals from the brain to optimize the weighting for combining signals from the same coil array elements for the low-concentrated 31P metabolites. 23Na-weighted Roemer combination of 31P MRSI signals is verified on EM simulations and MR experiments. Comparing to 31P self-weighted combination, 23Na-weighted combination shows higher SNR and better-combined spectra in regions with low intrinsic SNR. It also shows potential of mitigating the signal contaminations when using 31P-self-weights for 31P data acquired with large voxels.Introduction
31P MRSI is a powerful tool to study energy metabolism1 and cell proliferation and therefore being used in cancer treatment efficacy monitoring2. However, 31P MRSI is not widely used because of the low 31P SNR, (caused by low natural abundance and low gyromagnetic ratio compared to 1H). To harvest all potential SNR, some researchers use close-fit loop arrays for signal reception. However, the multi-channel signal combination3 can be challenging at low-SNR regions because coil sensitivity distributions are difficult to measure. Self-weighted techniques like WSVD4 combination and PCA based denoising have made significant progress in this area5. In this study, we explore the feasibility of another approach enabled by a coil array design which contains 15 loop-coils tuned to phosphorous, sodium and carbon6. We will investigate combining multi-channel 31P signals using the sensitivity distributions of 23Na which is expected to be similar because they share the same coil array geometry and the frequencies of operation of both nuclear species are still in near-field regime.Self-weighted Roemer combination method works reasonably well if SNR is sufficiently large (e.g. brain). However, when it comes to the body region, or regions surrounded by highly concentrated 31P signals (i.e. muscle), the low-SNR makes self-weighted combination more problematic, while on top of this the signals contain a significant portion of voxel bleeding from neighboring voxels and therefore no longer represent the coil sensitivity.
This study will investigate the potential for using 23Na sensitivity maps for signal combination of 31P MRSI. We will test the applicability by EM simulations and brain MRSI scans. As the self-weighted Roemer combination is still robust in most brain regions, our experiment with 23Na-weights can be validated.
Methods
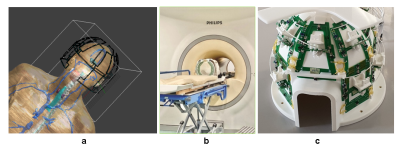
Simulations: We performed EM simulations on Sim4Life (Zurich Med Tech, CH). We simulated the B1- fields at the frequencies of 31P and 23Na on DUKE7. Figure 1a shows the simulated model. By approximating 31P signals with simulated B1-*, we compared self-weighted and 23Na-weighted Roemer combinations by evaluating the theoretical SNR. Equation 1 is used for SNR evaluation3, omitting all global scaling factors:$$SNR^2=\frac{(\omega MV)^2 \sum_{i=1}^N \sum_{k=1}^N n_i n_k B_{ti} B_{tk}}{4KT\Delta f \sum_{i=1}^N \sum_{k=1}^N n_i n_k R_{ik}\cos(\theta_i - \theta_k)} \qquad \text{(Eq1)}$$
MR experiments: The MR experiments were performed on a 7T MRI system (Philips Healthcare, Best, the Netherlands). An embedded-in-bore birdcage coil is used for 31P transmission. A Helmholtz coil is used for 23Na transmission. A quintuple-tuned head coil6 is used for reception. Figure 1b-c show the coil. 31P MRSI is acquired with the following specs: FOV = 320(FH)x260(AP)x240(RL) mm3, resolution = 20x20x20 mm3, TR = 300 ms, TE = 0.5 ms, FA = 20°, readout bandwidth=5000 Hz, 18 averages. 23Na MRI was acquired with the same resolution.
Data processing: Data processing is performed in Python for simulations and on MATLAB (The MathWorks, Inc.) for MR data. PCA-based denoising5 is performed after signal combination. Roemer’s optimal-SNR method is used for multi-channel signal combination (Eq2 below). We implement the 23Na weights by replacing the $$$b$$$ in Eq2 with 23Na MRI of identical resolution. The channel-wise constant 31P/23Na phase offset due to arbitrary phase shifts in the Rx chain is calibrated according to the 31P/23Na phase discrepancy at the maximum-23Na-signal locations.
$$P=C \frac{p^T R^{-1} b}{\sqrt{b^T R^{-1}b^*}} \qquad \text{(Eq2)}$$
Results
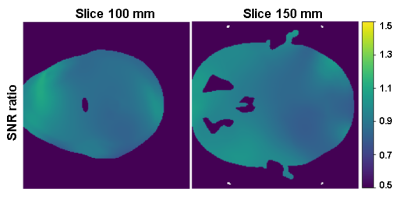
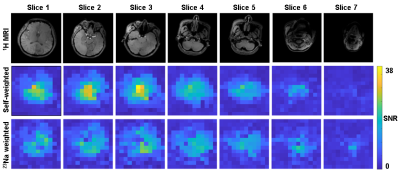
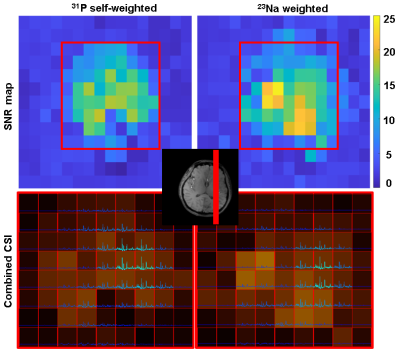
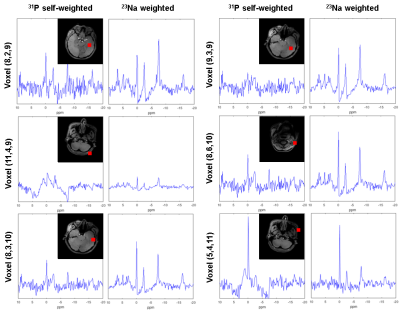
B1- maps comparison: Figure 2 shows the simulation-based comparison between the two combination methods on two sampled slices. The SNR can drop by 10% to 20% when using 23Na weights.In vivo MR: Figure 3 shows the SNR analysis of combined 31P MRSI from an in vivo scan. The second and the third row correspond to the SNR of the combined signals with 31P-self-weights and with 23Na weights respectively. Figure 4 compares these two methods by presenting the combined MRSI of slice 4. Figure 5 presents combined spectra comparisons of more sampled voxels.
Discussion
Simulations demonstrate that the weights for 31P coil element combinations can be correctly determined from 23Na signals of the same coil elements to maintain highest sensitivity. Also for most in vivo slices the 23Na weights are well assessed. However, looking at the first three columns of Figure 3, it seems like self-weighted Roemer combination generates undoubtably higher SNR for 31P MRSI. In fact, in center voxels the 23Na-weighted method shows almost no signal where self-weights combination shows outstanding SNR. It should be noted however that these center voxels are mainly in CSF where 31P concentration is low and sodium content high. Moreover due to the large voxels, there will be substantial point-spread contributions from higher signals from neighboring voxels towards the coils. Consequently, the observed signal will not provide the correct amplitude and phase weighting information for coil combinations. In the 31P-(self)-weighted reconstruction, SNR seems high, but this may be severely biased by optimizing the weights for the point-spread contribution. This is confirmed by substantial artefacts in 31P spectra from areas in the head close to tissue interfaces when using self-weighting while the spectra have good quality when using 23Na weighting.Conclusion
By using the same receive elements, 23Na-weighted Roemer combination of 31P CSI performs well in brain, and it even shows potential advantage over 31P-self-weights.Acknowledgements
Great thanks to Lieke van den Wildenberg and Ayhan Gursan for their guide in data precessing!References
[1] Chen C, Stephenson MC, Peters A, Morris PG, Francis ST, Gowland PA. 31P magnetization transfer magnetic resonance spectroscopy: assessing the activation induced change in cerebral ATP metabolic rates at 3 T. Magn Reson Med. 2018;79(1):22-30.
[2] van der Kemp WJM, Stehouwer BL, Luijten PR, van den Bosch MAAJ, Klomp DWJ. Detection of alterations in membrane metabolism during neoadjuvant chemotherapy in patients with breast cancer using phosphorus magnetic resonance spectroscopy at 7 Tesla. Springerplus. 2014;3(1):1-7.
[3] Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16(2):192-225.
[4] Rodgers CT, Robson MD. Receive array magnetic resonance spectroscopy: whitened singular value decomposition (WSVD) gives optimal Bayesian solution. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2010;63(4):881-891.
[5] Froeling M, Prompers JJ, Klomp DWJ, van der Velden TA. PCA denoising and Wiener deconvolution of 31P 3D CSI data to enhance effective SNR and improve point spread function. Magn Reson Med. 2021; 85: 2992– 3009. https://doi.org/10.1002/mrm.28654
[6] Dai J, Meliadò EF, Gosselink WJM, Arteaga C, Raaijmakers AJE, Klomp DWJ. Generalized approach of quadruple or quintuple-tuned RF coil setups for metabolic MRI throughout the body at 7 Tesla. ISMRM2022 Annual meeting, Abstract number 0711.
[7] Christ A, Kainz W, Hahn EG, et al. The Virtual Family—development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2009;55(2):N23.
Figures