3927
9 crossings per 1 bend: Tractography of contra- and ipsilateral connections in mouse optic pathways improved with ODF-Fingerprinting1Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, NYU Langone Health, New York, NY, United States, 2NeuroImaging and Visual Science Laboratory, Departments of Ophthalmology and Radiology, NYU Langone Health, New York, NY, United States, 3Department of Neurosurgery, Perlmutter Cancer Center, Neuroscience Institute, Kimmel Center for Stem Cell Biology, NYU Langone Health, New York, NY, United States, 4Radiological Sciences Laboratory and Molecular Imaging Program at Stanford, Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Image Reconstruction, White Matter, tractography, optic pathways, optic tract, optic chiasm, crossing fibers
Reconstruction of rodent optic pathways is particularly challenging for dMRI tractography due to the relatively small size of the crossing area in the optic chiasm and the unbalanced proportion between the contra- and ipsilateral axonal links. In this study, we replace the commonly used Orientation Distribution Function peak finding approach with our dictionary-based technique called ODF-Fingerprinting to improve the reconstruction of crossing fibers and thus correct the proportion of tracts exiting the optic chiasm. Our results from in vivo diffusion-weighted images of 18 mice show significant improvement (p<0.05) in many cases, helping decrease the discrepancies between reconstruction and gold-standard histology.Introduction
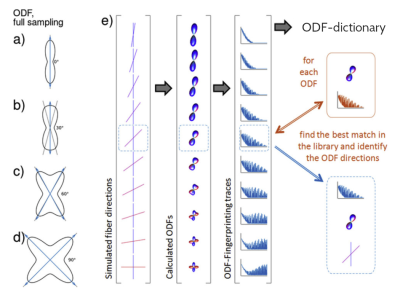
Axons in the mouse optic pathways cross and bend in the optic chiasm in the 9:1 proportion according to histology [1,2], hence 90% of the axons in optic tracts connect with the contralateral and 10% with the ipsilateral brain hemisphere. Even though diffusion MRI (dMRI) tractography allows for reconstruction of the optic pathways in vivo [2,3], the obtained ratio between streamlines in the contra- and ipsilateral pathways significantly deviates from the ground truth due to uncertainties in representation of the crossing area [2].This study aims to improve reconstruction of crossing fibers in the mouse optic chiasm by replacing the commonly used Orientation Distribution Function (ODF) peak finding approach with a dictionary-based technique called ODF-Fingerprinting (ODF-FP) [4,5] illustrated in Figure 1. For this, we consider in vivo diffusion-weighted images (DWIs) of 18 mice and quantify the proportions of contra- and ipsilateral connections reconstructed through deterministic tractography.
Our results show that ODF-FP significantly (p<0.05) outperforms the peak finding approach for detecting crossing fibers for rodent optic pathways. The observed improvement in a well-characterized in vivo structure with crossing fibers suggests ODF-FP may also reduce uncertainty of tractography in other brain areas with complex orientations of fiber bundles compared to other state-of-art methods.
Methods
Animals:This study was approved by the Institutional Animal Care and Use Committee. All 18 mice (genotype C57BL/6N) were females and 7-8 weeks old at the scan time.
Data:
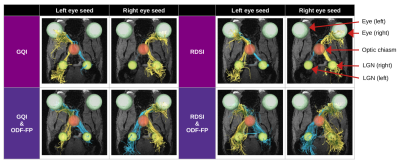
We acquired multishell DWIs sampled at 60 directions (distributed on radial lines at b=250,1000,2250,4000s/mm2 interleaved with 5 images at b=0) with 200x200x200µm resolution. We denoised our DWIs with the MRtrix3 dwidenoise [6] and then calculated ODFs using two reconstruction methods (independently), i.e. Generalized Q-sampling Imaging (GQI) [7] and Radial Diffusion Spectrum Imaging (RDSI) [8], to determine the impact ODF-FP has on different state-of-the-art algorithms. We then generated a randomized ODF-dictionary [5] of 106 elements containing 0≤N≤3 crossing fibers per voxel, with other parameters matching the dMRI acquisition. We applied ODF-FP to the ODF maps obtained with GQI and RDSI (separately) to reconstruct fiber directions. Having these, we ran deterministic tractography in DSI Studio [9] using fiber directions computed with each of the tested approaches. For each mouse, we defined 2 seeding regions located posterior to the globes and 3 regions of interest (ROIs) – one in the optic chiasm and two in the Lateral Geniculate Nuclei (LGNs) as shown in Figure 2. In deterministic tractography, we limited the number of seeds to 1000000, maximum angle to 60°, and Normalized Quantitative Anisotropy (NQA) to 0.01.
Evaluation:
We considered 4 variants of connections between the eyes and the brain hemispheres (Figure 2): Left to Left (L→L), Left to Right (L→R), Right to Left (R→L), and Right to Right (R→R). For each algorithm, we computed the proportion of contra- vs. ipsilateral links measured as numbers of streamlines originating at the respective seeding region, traversing the optic chiasm, and reaching the respective LGN. Similarly, we calculated connectivity measures normalized with median streamline lengths in DSI Studio and then compared the results using dependent t-test for paired samples.
Results
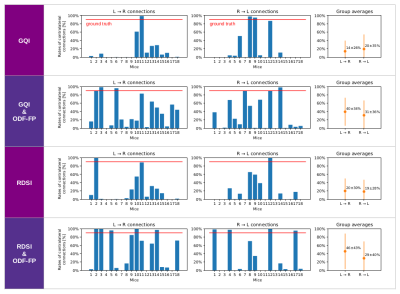
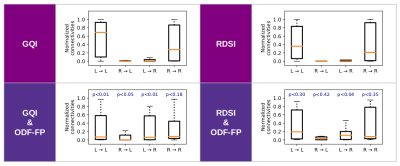
The use of ODF-FP visibly increased the number of streamlines connecting the optic nerves with the contralateral hemispheres, either in GQI or RDSI (Figure 2). In nearly all mice, we observed an improvement in proportions between contra- and ipsilateral links for both GQI&ODF-FP and RDSI&ODF-FP with respect to the original GQI and RDSI (Figure 3). Nonetheless, the ground truth level of approximately 90% was reached only in 3 out of 18 mice when using GQI&ODF-FP and in 5 mice with RDSI&ODF-FP.The normalized connectivity measures showed that the reconstruction based on the original peak finding methods produced strong ipsilateral links and weak contralateral ones, which contradicted gold-standard histology. Our proposed ODF-FP approach reduced ipsilateral connectivity and increased contralateral connectivity (Figure 4). This improvement was particularly visible in the GQI-based reconstruction (p<0.05) for all pairs of endpoints except for R→R.
Discussion
Reconstruction of rodent optic pathways is particularly challenging for dMRI tractography due to the relatively small size of the crossing area in the optic chiasm and the unbalanced proportion between the contra- and ipsilateral links. Moreover, the commonly used reconstruction techniques are frequently validated on human optic pathways, which – like in other primates – cross and bend in the optic chiasm at an approximately even proportion [2].We managed to alleviate these issues by improving the reconstruction of crossing fibers with ODF-FP that visibly altered the ratio of streamlines exiting the optic chiasm. However, our corrected proportions still deviated from the ground truth, calling for further investigation.
Our study showed that ODF-FP can be applied to different reconstruction algorithms (like GQI or RDSI) leading to different levels of improvement. Future work should address the use of ODF-FP with alternative algorithms and possibly also with the probabilistic tractography. An additional improvement would likely arise from increasing the in-plane resolution of DWIs, thus helping to augment the fiber crossing area.
Acknowledgements
This project is supported in part by the National Institutes of Health (NIH, R01-EB028774 and R01-NS082436). It was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, https://www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41-EB017183), and at the NYU Langone Health Preclinical Imaging Laboratory, a shared resource partially supported by the NIH/SIG 1S10OD018337-01, the Laura and Isaac Perlmutter Cancer Center Support Grant NIH/NCI 5P30CA016087 and the NIBIB Biomedical Technology Resource Center Grant NIH P41-EB017183References
[1] Forrester et al., Nerve fibres in the optic nerve of rat, Nature, vol. 214, 1967.
[2] Deng et al., Applications of manganese-enhanced magnetic resonance imaging in ophthalmology and visual neuroscience, Frontiers in Neural Circuits, vol. 13, 2019.
[3] Xu et al., Assessing optic nerve pathology with diffusion MRI: from mouse to human, NMR in Biomedicine, vol. 21, 2008.
[4] Baete et al., Fingerprinting Orientation Distribution Functions in diffusion MRI detects smaller crossing angles, Neuroimage, vol. 198, 2019.
[5] Filipiak et al., Performance of orientation distribution function‐fingerprinting with a biophysical multicompartment diffusion model, MRM, vol. 88(1), 2022.
[6] Veraart et al., Denoising of diffusion MRI using random matrix theory, NeuroImage vol. 142, 2016.
[7] Yeh et al., Generalized q-sampling imaging, IEEE TMI, vol. 29, 2010.
[8] Baete et al., Radial q-space sampling for DSI, MRM, vol. 76, 2016.
[9] Yeh, http://dsi-studio.labsolver.org/
Figures