3925
Feasibility of simultaneous fMRI and optical imaging for head-fixed freely-behaving mice1Emory University/Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
Keywords: Multimodal, Brain
For simultaneous optical imaging and fMRI with freely-behaving mice, head-fixed without body restriction in minimal stress awake state, optimizations were conducted on multiple aspects, including multi-wavelength optical imaging system for both optical intrinsic signals and extrinsic fluorescence studies of genetically encoded calcium indicators (GECI) and voltage indicators (GEVI) mice with tube lens design instead of imaging fiber bundle, transparent cranial window development, integrated quadrature coil in head holder, and the designed belt treadmill/ virtual reality (VR) system to fit a customized rodent cradle in the commonly used 12cm ID gradient for the small animal MRI system.INTRODUCTION
Neuroimaging studies in awake rodents with head fixation but without other body restriction are ideal for translational studies of behavior while minimizing stress1,2. This study paradigm is well-established for bench-top electrophysiological recording and optical imaging, where immobilization of the head is an imaging requirement but the relative freedom of the rest of the body allows close-to-natural behavior, i.e. running on a treadmill during virtual reality (VR) exploration3,4. In our preliminary studies, we have developed a method for integrating optical imaging with fMRI in rodent to take advantage of both the cell type-specificity of state-of-the-art optical fluorescence cortical imaging techniques and the whole brain coverage of fMRI studies5. In this report, we present our further strategies for development of simultaneous optical imaging and fMRI for freely-behaving mice.METHODS
Tube lens method for optical camera imaging during fMRI: We set a multi-camera system outside the magnet room and used a 4.5-m long tube lens to access a mouse cortex window for optical imaging during fMRI when the animal set in a customized cradle5. Both genetically encoded calcium indicators (GECI) and voltage indicators (GEVI) mice (n=6) were tested and validated for image quality in our simultaneous multiple-wavelength imaging setup, which provides flexible illumination and detection of blue, green, red and NIR light. For example, we use 100Hz frame rate with 100*100 pixel resolution when simultaneously imaging fluorescent signals at 525/50 nm and reflectance measurements of intrinsic signals at the same wavelength for hemodynamic signal separation.Transparent optical window and coil integration method: We used a thinned skull procedure and applied optical adhesive to result in transparent bone over the cortex. A 3D-printed headpiece was attached on the skull with dental cement in advance of the skull thinning procedure, Figure 1. An additional 3D-printed holding piece was used to fit the headpiece with a quick-release design. An anatomically-fitted single loop surface coil or quadrature coil was designed and integrated in the head holding piece for transmit/receive RF signals. For the quadrature coil, the orthogonal combination is one single loop overhead and another beside the mouse brain in a coil of two loops. The mouse head was fixed into place with the headholder.
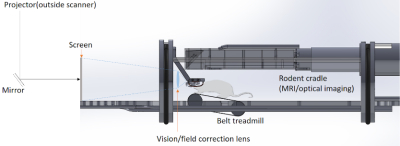
Simultaneous fMRI and optical imaging in freely-behaving mice: A small belt treadmill was designed to fit the mouse body and the cradle for the 12-cm gradient bore. The belt treadmill is tilted 10 degrees to facilitate animal running. To obtain a large field of view (~90 degree) for VR navigation, a corrective lens is set in front of the eyes before a projected screen, figure 2.
RESULTS
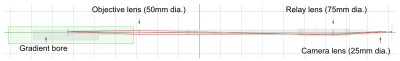
In our optimized design, we combined 3 different size lens with broad-band coating (400-1000 nm) in a 4.5-m optic path, Figure 3. The total 3 lens transmission efficiency is better than 98% (each has ~0.5% reflection). The 50mm-dia. objective lens was set in ~80cm working distance for 8mm*8mm mouse cortex (~0.032 NA).Our novel crystal optical window method was successfully applied in 4 of 6 animals. Two of the animals during the initial development had bubbles but solved in the other 4 animals using a cover glass cover slightly less than the window area. The optical image quality is satisfactory, with clear vessel-parenchyma contrast. Either a heated-water circulation bed (for anesthetized animals) or a tilted belt treadmill (for awake animals) can be fitted into the cradle within the 12cm-ID gradient bore.
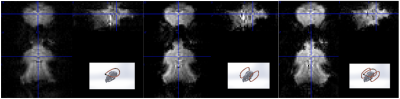
We compared loop coils of 0 degree, 90 degree and quadrature setup in the anatomically fitted designs. The GE-EPI scans demonstrated a better signal sensitivity and homogeneity for whole brain coverage using the quadrature setup, Figure 4, 330um isotropic voxels and 125ms/15ms TR/TE in 9.4T.
DISCUSSION/CONCLUSION
Multi-modality neuroimaging has been a significant direction for preclinical studies, combining the strengths of cell-specific recording with the whole brain coverage of fMRI. The major challenge when combining optical imaging and fMRI is to ensure optical image quality, since bulky scientific-grade cameras have to be set several meters away from magnet. Meanwhile, freely behaving mice setup would be ideal for minimal restriction-induced stress and close-to-natural performance of exploration tasks in experiments. We have optimized multiple aspects of the studies, from the optical design, the cortical window, and the anatomy-conforming coil design to the treadmill for navigation in VR. We demonstrated a cost-efficient tube lens method instead of using expensive image fiber bundles, which could pave the way for wider application. The latter may set in short working distance to achieve a higher NA6, but the image fiber bundle has limited transmission efficiency (40%) and limited wavelength range (>500nm). The overall optical transmission efficiency should be comparable without the budget for an expensive image fiber bundle. For freely behaving mice studies with the treadmill/VR system, we designed a properly sized belt treadmill to replace the heating bed when switching from an anesthetized rodent study. To achieve a maximum view of field, a correction lens for viewing a frontal screen will be necessary. Our initial efforts to modify the system to fit within a commonly used 12-cm gradient bore of small-animal MRI system paves the way to adding a new dimension in upcoming studies that will benefit preclinical neuroscience research.Acknowledgements
This work was supported by NIH grants: r01mh111416, r01ns078095, and r01eb029857.References
1. West, S. L. et al. Wide-Field Calcium Imaging of Dynamic Cortical Networks during Locomotion. Cerebral Cortex 32, 2668–2687 (2022).
2. Paasonen, J. et al. Whole-brain studies of spontaneous behavior in head-fixed rats enabled by zero echo time MB-SWIFT fMRI. NeuroImage 250, 118924 (2022).
3. Thurley, K. & Ayaz, A. Virtual reality systems for rodents. Curr Zool 63, 109–119 (2017).
4. Prince, S. M. et al. Alzheimer’s pathology causes impaired inhibitory connections and reactivation of spatial codes during spatial navigation. Cell Reports 35, (2021).
5. Pan, W.-J. et al. (ISMRM 2022) Optimization of wide-field optical imaging method towards fMRI integration in mice. https://archive.ismrm.org/2022/3331.html.
6. Lake, E. M. R. et al. Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI. Nature Methods 17, 1262–1271 (2020).
Figures