3921
Exercise and Environmental Complexity Superintervention Improves Mechanical Property Recovery in FASD Rodents Measured via MRE1Dept. of Biomedical Engineering, University of Delaware, Newark, DE, United States, 2Dept. of Psychological & Brain Sciences, University of Delaware, Newark, DE, United States, 3Laboratorie de Mécanique et Génie Civil, CNRS, Université de Montpellier, Montpellier, France, 4Département de Génie Mécanique, Université de Sherbrooke, Sherbrooke, QC, Canada, 5Thayer School of Engineering, Dartmouth College, Hanover, NH, United States
Synopsis
Keywords: Elastography, Preclinical
In this longitudinal study, we investigate the effects of fetal alcohol exposure on mechanical properties in the developing rodent brain using magnetic resonance elastography (MRE). Additionally, we test whether behavioral “superintervention,” consisting of combined exposure to wheel running followed by environmental complexity, can mitigate changes to property measures. We found that alcohol exposed rats exhibit lower stiffness than sham intubated rats at baseline but recover stiffness to near baseline levels after superintervention. Furthermore, we confirmed MRE is sensitive to small property variations in rodents which may reflect myelination and microstructural integrity.Introduction
Fetal alcohol spectrum disorders (FASDs) can result from alcohol exposure (AE) during the prenatal period. Individuals diagnosed with FASDs suffer from impaired cognition, motor control, emotionality, and social behavior.1 Neuroanatomical changes brought on by AE include abnormal myelination and white matter connectivity, particularly in executive function-related neural circuitry.2 Behavioral treatments for AE are nonexistent, but exercise has been shown to enhance proliferation of neuronal precursors and increase white matter growth.3,4 Furthermore, environmental complexity following exercise extends the survival of newly generated cells in rats.5 Brain mechanical properties measured in vivo using magnetic resonance elastography (MRE) may reflect these microstructural changes in FASD rats.MRE is a noninvasive, quantitative MRI technique used to estimate mechanical properties. While preclinical MRE research is limited,6,7 we validated a technique for rodent MRE using the nonlinear inversion algorithm (NLI) with translatable quality and resolution to humans.8 In this longitudinal study, we investigate the effects of fetal alcohol exposure on mechanical properties in the developing rodent brain. Additionally, we test whether behavioral “superintervention,” consisting of combined exposure to wheel running (WR) followed by environmental complexity (EC), can mitigate AE-induced changes to MRE measures.
Methods
Animal Preparation & ImagingTo model FASD, female Long Evans rat pups (n=9) were given alcohol (5.25 g/kg) in milk formula twice per day from postnatal days (PD) 4-9 via intragastric intubation. Sham intubated (SI) controls were not administered any liquid during intubation (n=11). All rats were weaned and socially housed at PD23.
Between PD25-29, MRE and DTI scans were performed on animals anesthetized with 1-3% isoflurane using a Bruker 9.4T preclinical imaging system. For the MRE scans, we used a nonmagnetic, piezoelectric actuator and bite bar driver to apply vibrations at 800 Hz. A resolution of 0.25x0.25x0.5 mm3 was achieved in 32 minutes using a custom MRE-EPI sequence with frequency matched motion encoding gradients with the following imaging parameters: TE/TR = 60/3400 ms, FOV = 20x20 mm2, matrix = 80x80, slice thickness = 0.5 mm, slices = 40, averages = 24. The MRE scan was followed by a 30 direction diffusion tensor imaging scan which is required to measure anisotropy.
Following the first scan (TP1), half of the rats were assigned to the wheel running/environmental complexity (WR/EC) superintervention group. They were pair-housed in cages with free access to an attached running wheel where they could exercise for 12 days (PD30-42). WR/EC rats were then transferred to environmental complexity cages (6-9 animals/cage). These cages were furnished with various objects and toys that were changed every other day. The remaining rats were returned to social housing (SH). After 30 days, all rats received their second MRE and DTI scans (TP2). Figure 1 shows the timeline of this study.
Mechanical Property Estimation
Mechanical properties were estimated from MRE displacement data using isotropic NLI with a no boundary condition formulation (NoBC-NLI).9 NLI outputs storage modulus (G’) and loss modulus (G’’) which were used to calculate shear stiffness (μ). We also performed preliminary inversions using transversely isotropic NLI (TI-NLI), which provides more information about anisotropic tissues (such as white matter), by estimating 3 parameters: baseline shear stiffness (μ), shear anisotropy (φ), and tensile anisotropy (ζ), where φ and ζ represent higher stiffness in the fiber direction.10 Primary fiber directions are given by the DTI data.
Results & Discussion
Figure 2 shows AE rats exhibited softer brain tissue than SI controls at TP1 (SI = 6.59 kPa, AE = 5.94 kPa, p = 0.012); this stiffness difference likely reflects lower white matter integrity in AE rats. Preliminary results from TI-NLI suggest that shear anisotropy and tensile anisotropy are also lower in AE rats compared to controls (Figure 3); however, optimization of TI-NLI for rodent MRE is needed to confirm how the anisotropic properties are affected. While the AE rats had slightly lower stiffness than SI rats at TP2 (SI = 6.84 kPa, AE = 6.46 kPa, p = 0.069), this difference was less than at TP1, suggesting that the superintervention led to stiffness recovery. Indeed, the longitudinal analysis (Figure 4) indicated there was no difference from TP1 to TP2 for SI (Δμ = 0.26 kPa, p = 0.32), though the AE group saw a significant increase in stiffness over time (Δμ = 0.52 kPa, p = 0.020). The WR/EC condition also had a positive effect on stiffness (Δμ = 0.45 kPa, p = 0.010) which agrees with the hypothesis that superintervention proliferates neuronal growth; there was also no significant difference for the SH control condition (Δμ = 0.28 kPa, p = 0.39). This conclusion was reinforced by a significant increase in stiffness only within the AE/WR/EC subgroup (Δμ = 0.62 kPa, p = 0.029).Conclusion
This study demonstrates that exercise followed by environmental complexity has a positive effect on rodent brain mechanical properties. The superintervention particularly improved the stiffness of alcohol exposed rats over time, such that brain stiffness recovered to near baseline values. Furthermore, we confirmed MRE is sensitive to small property variations in rodents which may reflect myelination and microstructural integrity. In future work, we will correlate these results with immunohistochemistry and investigate how fetal alcohol exposure and superintervention impact anisotropic properties in white matter throughout development.Acknowledgements
NIH/NIBIB R01 EB027577 (Johnson), NIH/NIAAA R01AA027269-01 (Klintsova), NIH 2P20GM10365 (University of Delaware Center for Biomedical and Brain Imaging; Klintsova and Johnson)
References
1. Streissguth, A. P., Aase, J. M., Clarren, S. K., Randles, S. P., LaDue, R. A., and Smith, D. F. (1991). Fetal alcohol syndrome in adolescents and adults. JAMA J. Am. Med. Assoc. 266, 1077.
2. Wilhelm, C. J., and Guizzetti, M. (2016). Fetal alcohol spectrum disorders: An overview from the glia perspective. Front. Integr. Neurosci. 9, 1–16.
3. Helfer, J. L., Goodlett, C. R., Greenough, W. T., and Klintsova, A. Y. (2009). The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res. 1294, 1–11.
4. Milbocker, K. A., LeBlanc, G. L., Brengel, E. K., Hekmatyar, K. S., Kulkarni, P., Ferris, C. F., et al. (2022). Reduced and delayed myelination and volume of corpus callosum in an animal model of Fetal Alcohol Spectrum Disorders partially benefit from voluntary exercise. Sci. Rep. 12, 1–17.
5. Hamilton, G. F., Boschen, K. E., Goodlett, C. R., Greenough, W. T., and Klintsova, A. Y. (2012). Housing in Environmental Complexity Following Wheel Running Augments Survival of Newly Generated Hippocampal Neurons in a Rat Model of Binge Alcohol Exposure During the Third Trimester Equivalent. Alcohol. Clin. Exp. Res. 36, 1196–1204.
6. Bayly P V., Garbow JR. Pre-clinical MR elastography: principles, techniques, and applications. J Magn Reson. 2018;291:73-83.
7. Bigot M, Chauveau F, Beuf O, Lambert SA. Magnetic resonance elastography of rodent brain. Front Neurol. 2018;9(NOV):1-8.
8. Williams LT, Milbocker KA, Sullivan SR, et al. Mapping Rodent Brain Mechanical Properties In Vivo with Magnetic Resonance Elastography and Nonlinear Inversion. In: Proc. Intl. Soc. Mag. Reson. Med. 2022:10-12.
9. McGarry MDJ, Van Houten EEW, Johnson CL, et al. Multiresolution MR elastography using nonlinear inversion. Med Phys. 2012;39(10):6388-6396.
10. McGarry M, Van Houten E, Sowinski D, et al. Mapping heterogenous anisotropic tissue mechanical properties with transverse isotropic nonlinear inversion MR elastography. Med Image Anal. 2022;78:102432.
Figures
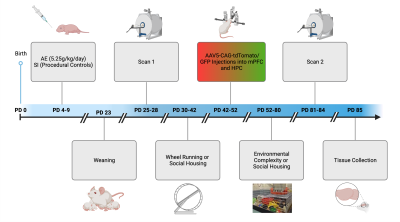
Figure 1. Experiment timeline. Note that the treatment conditions (SI and AE) are established at PD4-9, prior to scan one (TP1) while the intervention conditions (SH and WR/EC) are established at PD30-42, prior to scan two (TP2). Immunohistochemistry and viral axon tracing will be covered in future work.
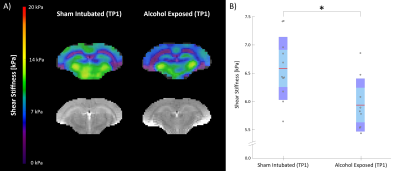
Figure 2. (A) Representative shear stiffness maps for sham intubated (SI) and alcohol exposed (AE) rats at scan one (TP1). (B) Shear stiffness results from TP1. AE rats are significantly softer than SI rats (p = 0.012).
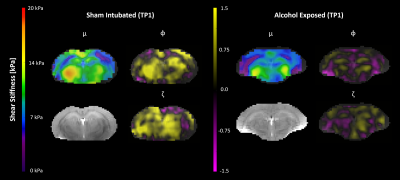
Figure 3. Representative property maps of baseline shear stiffness (μ), shear anisotropy (φ), and tensile anisotropy (ζ) from sham intubated (SI) and alcohol exposed (AE) rats at scan one (TP1). AE rats exhibit lower anisotropic properties compared to SI rats.
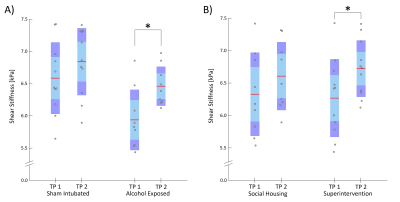
Figure 4. (A) Shear stiffness results for sham intubated (SI) and alcohol exposed (AE) rats from scan one (TP1) to scan two (TP2). Shear stiffness increased significantly in AE rats (p = 0.020). (B) Shear stiffness results for the social housing (SH) and wheel running/environmental complexity superintervention (WR/EC) groups from TP1 to TP2. Shear stiffness increased significantly in the WR/EC group (p = 0.010).