3920
Development and application of dynamic MRSI of an HP neuroprotective agent in an MCAO mouse model of ischemic stroke at 14.1 T1Laboratory of Functional and Metabolic Imaging, École polytechnique fédérale de Lausanne (EPFL), Lausanne, Switzerland, 2Geneva School of Health Sciences, HES-SO University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland, 3Department of Clinical Neurosciences, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 4CIBM Center for Biomedical Imaging, École polytechnique fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5Department of Health Technology, Center for Hyperpolarization in Magnetic Resonance, Technical University of Denmark, Kgs Lyngby, Denmark, 6Image Guided Intervention Laboratory, University of Geneva (UNIGE), Geneva, Switzerland
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Preclinical, Molecular Imaging
Advanced acquisition hardware and sequences boosting the spatiotemporal resolution overcome the existing limitations of HP metabolic MRI in animal disease models, providing new contrasts and opening up new perspectives. Herein, we designed a 1H/13C volume/surface-combined head coil providing full mouse brain coverage with high sensitivity, as well as a high-efficiency acquisition scheme to achieve superior spatial and temporal resolution images. This setup was employed for time-resolved imaging of the cerebral metabolic response to a neuroprotective bolus of hyperpolarized pyruvate in a transient hypoxia/ischemia mouse model. The experiments suggest a distinct metabolism between the ischemic and healthy tissues.
Introduction
With no doubt, dynamic nuclear polarization (dDNP) is among the most performant and versatile hyperpolarization (HP) techniques providing high sensitivity probes.Distinct metabolism of HP neuroprotective agents such as lactate2 or pyruvate3 injected at a therapeutic dose into mice subjected to ischemic stroke were found with a dynamic global acquisition scheme. However, disentangling the specific regional metabolic patterns resulting from a stroke injury requires appropriate localized measurements. Better sensitivity and spatiotemporal resolution achieved using state-of the art acquisition hardware and sampling schemes have the potential to enable the observation of new contrasts, therefore providing new insights into the rapidly-evolving cerebral metabolism following ischemia.
Here, expanding upon previous works4,5, we designed a new RF coil setup improving the mouse brain coverage and strengthened the robustness of the acquisition scheme to achieve time-resolved HP magnetic resonance spectroscopy imaging (MRSI) in the same mouse model at ultrahigh field.
Methods
MR measurements were performed on a 14.1T/260mm magnet, 1T/m 6450T/m/s shielded gradient set (BFG240-120-S12B) and a BioSpec Advance NEO spectrometer (Bruker).Radio-frequency (RF) coil setup:
A 1H/13C transmit/receive (Tx/Rx) volume coil with two orthogonal saddle coils and a 13C receive-only (Rx-only) 2-loop surface coil were designed for the mouse head (Fig.1A-B). 13C gradient echo in 5mm axial slices were acquired in a cylindrical 0.4M [1-13C]acetate phantom to map the transmit field (B1+) amplitude using the double angle method6 and the receive sensitivity.
Imaging sequence:
A multi-slice IDEAL Spiral CSI sequence7 was used for HP MRSI. The gradient frequency was lowered to 500Hz to reduce gradient imperfections affecting the measurement of large bolus signals. The sequence was tested in three contiguous 4mm axial slices in a 4-compartment phantom. For each slice, a spectrum was acquired with a slice-selective 5° Shinnar-Le Roux (SLR) pulse (BW=4200Hz), then seven shifted echo-time images (TE0=1.196ms, ∆TE=0.23ms) were measured with a 10° SLR pulse (BW=4200Hz), and single-shot spiral readout (20.65ms/2065pts, nominal 20x20 matrix, 20x20mm2 FOV). 256 averages with TR=2s were acquired.
MRSI after middle cerebral artery occlusion (MCAO) in mice:
[1-13C]pyruvic acid doped with 15mM OX063 was hyperpolarized at 5T/1.2K as described elsewhere5. In parallel, transient ischemic stroke was surgically induced in one C57BL6/J male mouse (8 weeks, 25.9g) using the MCAO model8,9.
At 1.5h post-reperfusion, the DNP sample was dissolved and transferred to a separator/infusion pump10. A therapeutic bolus9 (1.04µmol/g) of HP pyruvate (325µl, 80mM, ~30% polarization) was injected into the femoral vein.
Dynamic MRSI with identical parameters as above was automatically started for 60 repetitions with TR=2s. The Tx/Rx frequencies were centered on [1-13C]pyruvate.
Results
RF coil:The B1+ amplitude map depicts a homogeneous transmit field (Fig.1C), with a 3.0% coefficient of variation (COV) within a 10mm×6mm ellipse (roughly the largest mouse brain axial cross-section). Receive sensitivity maps (Fig.1D-F) depict a full but inhomogeneous brain coverage, with a 21.3% COV in the combined image.
Phantom measurements:
13C spectroscopic images of the distinct compartments overlaid on proton images (Fig.2) confirm that proper spatial localization was achieved.
MRSI in an MCAO mouse:
Dynamic slice-selective spectra (Fig.3) acquired prior to spiral-encoded images depict the arrival of pyruvate and its conversion into lactate. The ischemic lesion is located in the striatum, on the right-hand side of the anterior slice. Dynamic (Fig.4A-C) and time-averaged (Fig.5A) spectroscopic images depict pyruvate both within and around the brain, while lactate is predominantly detected within the brain. Overall, lower 13C signals are measured in the ipsilateral hemisphere (Fig.4D-F, Fig.5A-B).
On the anterior slice, the lactate-to-pyruvate ratio (LPR) rises faster and earlier in the ipsilateral side (Fig.4D). Time-averaged maps (Fig.5) show a higher LPR in the ipsilateral side, specifically within the ischemic lesion. In the central and posterior slices, which have no lesions, similar LPR levels and dynamics are observed on either side (Fig.4E-F, Fig.5).
Discussion
The RF coil setup provided a homogeneous excitation profile and full coverage of the brain. Thanks to improvements in the polarization procedure5, dynamic MRSI was successfully achieved in the ischemic mouse brain.Following a bolus of HP [1-13C]pyruvate, its uptake and metabolism into [1-13C]lactate were measured. In the ipsilateral hemisphere, lower signals of both pyruvate and lactate were observed, which could result from a lower blood perfusion resulting from the surgery, similarly to a previous report11. The LPR is higher in the region of the ischemic injury compared to contralateral tissues, in coherence with another study12. This could potentially indicate a higher pyruvate uptake due to increased transporter expression13, or higher LDH activity after ischemia14.
Our measurements face several inherent challenges of hyperpolarized MRS(I) at ultra-high field. Firstly, at higher magnetic field strengths, the longitudinal relaxation time of common HP metabolites decreases15, limiting our time window for imaging to 60s. Secondly, higher field strengths exacerbate static field inhomogeneities which are not well handled by the IDEAL approach. However, the chemical-shift reconstruction could exploit 1H field maps16 to correct these discrepancies.
Conclusion
RF coils were designed to improve acquisition sensitivity and coverage, leading to better performance of the IDEAL spiral CSI sequence. The time-resolved metabolism of HP pyruvate in an ischemic mouse brain was successfully imaged at a millimetric resolution every 2s for one minute. These preliminary results illustrate higher and faster lactate labelling in the ischemic region.Acknowledgements
This work was generously supported by the Swiss National Science Foundation (170155 to Jean-Noël Hyacinthe and Lorenz Hirt, 190547 and 193276 to Andrea Capozzi). The authors gratefully thank Prof. Rolf Gruetter for supporting this collaboration, Dr. Juan Diego Sanchez for fruitful discussions about RF coils, Dr. Analina Hausin and Dr. med. vet. Stefan Mitrea for their assistance in the animal preparation, as well as the CIBM Center for Biomedical Imaging, co-founded and supported by Lausanne University Hospital, University of Lausanne, École polytechnique fédérale de Lausanne, University of Geneva and Geneva University Hospitals.References
1. Ardenkjær-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100(18):10158-10163. doi:10.1073/pnas.1733835100
2. Hyacinthe JN, Buscemi L, Lê TP, Lepore M, Hirt L, Mishkovsky M. Evaluating the potential of hyperpolarised [1-13C] L-lactate as a neuroprotectant metabolic biosensor for stroke. Scientific Reports. 2020;10(1):5507. doi:10.1038/s41598-020-62319-x
3. Lê TP, Buscemi L, Vinckenbosch E, et al. Metabolism of the hyperpolarized neuroprotective agents [1-13C] lactate and [1-13C] pyruvate in a mouse model of transient ischemic stroke. In: Proc. ISMRM. ; 2020:689.
4. Lê TP, Capozzi A, Hyacinthe Jean-Noël. Hyperpolarized in-vivo Metabolic Imaging at 14.1T: dDNP Cryogenic Insert Redesign and Implementation. In: Proc. ISMRM. ; 2021:3804.
5. Lê TP, Hyacinthe JN, Capozzi A. How to improve the efficiency of a traditional dissolution dynamic nuclear polarization (dDNP) apparatus: Design and performance of a fluid path compatible dDNP/LOD-ESR probe. Journal of Magnetic Resonance. 2022;338:107197. doi:10.1016/j.jmr.2022.107197
6. Insko EK, Bolinger L. Mapping of the Radiofrequency Field. Journal of Magnetic Resonance, Series A. 1993;103(1):82-85. doi:10.1006/jmra.1993.1133
7. Wiesinger F, Weidl E, Menzel MI, et al. IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C]pyruvate. Magnetic Resonance in Medicine. 2012;68(1):8-16. doi:10.1002/mrm.23212
8. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84-91. doi:10.1161/01.STR.20.1.84
9. Castillo X, Rosafio K, Wyss MT, et al. A Probable Dual Mode of Action for Both L- and D-Lactate Neuroprotection in Cerebral Ischemia. J Cereb Blood Flow Metab. 2015;35(10):1561-1569. doi:10.1038/jcbfm.2015.115
10. Comment A, van den Brandt B, Uffmann K, et al. Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn Reson. 2007;31B(4):255-269. doi:10.1002/cmr.b.20099
11. Peeters TH, Kobus T, Breukels V, et al. Imaging Hyperpolarized Pyruvate and Lactate after Blood–Brain Barrier Disruption with Focused Ultrasound. ACS Chem Neurosci. 2019;10(5):2591-2601. doi:10.1021/acschemneuro.9b00085
12. Xu Y, Ringgaard S, Mariager CØ, et al. Hyperpolarized 13C Magnetic Resonance Imaging Can Detect Metabolic Changes Characteristic of Penumbra in Ischemic Stroke. Tomography. 2017;3(2):67-73. doi:10.18383/j.tom.2017.00106
13. Rosafio K, Castillo X, Hirt L, Pellerin L. Cell-specific modulation of monocarboxylate transporter expression contributes to the metabolic reprograming taking place following cerebral ischemia. Neuroscience. 2016;317:108-120. doi:10.1016/j.neuroscience.2015.12.052
14. Sapir G, Shaul D, Lev-Cohain N, Sosna J, Gomori MJ, Katz-Brull R. LDH and PDH Activities in the Ischemic Brain and the Effect of Reperfusion—An Ex Vivo MR Study in Rat Brain Slices Using Hyperpolarized [1-13C]Pyruvate. Metabolites. 2021;11(4):210. doi:10.3390/metabo11040210
15. Keshari KR, Wilson DM. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem Soc Rev. 2014;43(5):1627-1659. doi:10.1039/C3CS60124B
16. Reeder SB, Brittain JH, Grist TM, Yen YF. Least-squares chemical shift separation for 13C metabolic imaging. Journal of Magnetic Resonance Imaging. 2007;26(4):1145-1152. doi:10.1002/jmri.21089Figures
Fig.1: (A) 1H/13C volume coil. (B) 13C receive-only (Rx-only) coil for the mouse brain. The Rx-only coil has two actively detuned loops covering a 15x25mm2 curved surface (8mm radius) connected to two preamplifiers (G=24.9dB, NF=0.53dB at 150MHz). (C) 13C B1+ amplitude map normalized to the nominal flip angle in a 5mm axial slice. The bottom is not reliable due to limited receive coverage. The gray contour delimits the ellipse used to characterize the homogeneity. (D) Sensitivity map of the Rx-only coil, normalized to the effective flip angle. (E-F) Individual sensitivity maps.
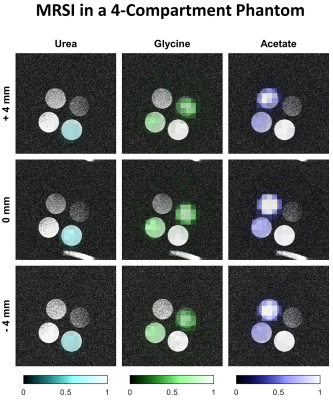
Fig.2: Multislice MRSI acquired in a phantom using the IDEAL spiral CSI sequence. In clockwise order from the uppermost compartment, the tubes contain water solutions with 10 mM Gd-DO3A-butrol and either sodium [1-13C] acetate 0.4M, [1-13C] glycine 0.4M, [13C] urea 0.4M, or both sodium [1-13C] acetate 0.2M and [1-13C] glycine 0.2M.
Fig.3: Dynamic slice-selective cerebral 13C spectra (magnitude, lb=25Hz) acquired after a bolus infusion of [1-13C] pyruvate, before each train of spectroscopic images. The sum is plotted in red. The same scale was applied to all slices. HP pyruvate converts into lactate. No alanine nor bicarbonate were observed. In the anterior slice, the multiple pyruvate (-hydrate) peaks likely result from B0 inhomogeneities. The corresponding anatomical slices are displayed under each set of dynamic spectra. The ischemic lesion (red contour) is located in the anterior slice.
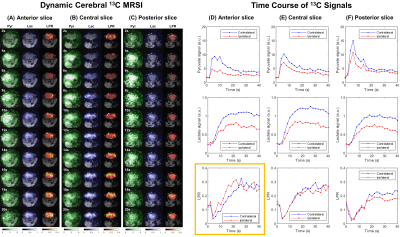
Fig.4 (A-C) Dynamic cerebral MRSI in an MCAO mouse after a bolus of HP pyruvate. Due to limited space, maps of pyruvate hydrate and repetitions after 20s are not shown. No zero-filling or masking were applied on metabolite maps. The voxel-wise lactate-to-pyruvate ratio (LPR) is reported within the brain. For each slice, metabolite signals and ratio time courses were quantified within either brain side (D-F). Overall, lower signals are measured in the ipsilateral hemisphere. In the anterior slice, where the lesion is located, the LPR rises faster in the ipsilateral side (D highlighted).