3919
First neuroimaging experience at 11.7 Tesla on the anaesthetized non-human primate.
Fawzi Boumezbeur1, Alexis Amadon1, Marion Gay1, Franck Mauconduit1, Vincent Gras1, Maxime Roustan1, Frederic Lepretre1, Aurélien Massire2, Cécile Rabrait-Lerman1, Sebastien Mériaux1, Alexandre Vignaud1, and Nicolas Boulant1
1NeuroSpin, CEA, Gif-sur-Yvette, France, 2Siemens Healthcare SAS, Saint-Denis, France
1NeuroSpin, CEA, Gif-sur-Yvette, France, 2Siemens Healthcare SAS, Saint-Denis, France
Synopsis
Keywords: Bioeffects & Magnetic Fields, High-Field MRI
After more than 20 years of research and development, the 11.7T Iseult MRI scanner is in the last stage of its commissioning. However, authorization of Human MRI examination at this unprecedented magnetic field strength is still pending. In this context, MRI examinations of healthy anaesthetized non-human primates are being carried out to check on the absence of acute physiological effects following exposure and gain some insights into the opportunities and challenges ahead.Introduction
Ultra-high magnetic fields (UHF) hold the promise of ever larger signals, contrasts and spectral resolution, pushing the limits of anatomical, functional and metabolic MRI and MRS. After more than 20 years of research and development, the 11.7T Iseult MRI scanner[1] is in the last stage of its commissioning. However, authorization of Human MRI examination at this unprecedented magnetic field strength is still pending. In this context, our first in vivo MRI and MRS examinations were performed at 11.7T on three healthy anaesthetized non-human primates to check on the absence of acute physiological effects following exposure and gain some insights into the opportunities and challenges ahead.Methods
Animals: Three male adult monkeys (macaca mulatta, body weight ~10 kg) were examined at 11.7T for 90 minutes in the supine position. All experimental procedures were approved by the regional ethics committee and performed in strict accordance with the European Communities Council Directive (EC/2010/63). Animals were anaesthetized first with a mixture of Ketamine (3 mg/kg im) and Dexdomitor (0.015 mg/kg im) and maintained using isoflurane at 1.5% in an O2/Air mixture. Cardiac and respiratory rates, blood oxygen saturation and body temperature were monitored during the experiments to ensure their physiological stability. Body temperature was maintained by a heated water circulating system.MR Sequences: In vivo MRI and MRS data were acquired using a multi-transmit coil developed in-house for human neuroimaging at 11.7 T (Fig. 1) [2]. This coil was operated in its default Tx-combined mode (pseudo-CP mode) for all but one acquisition: our last GRE sequence used dynamic parallel transmission (pTx) to recover some signal loss from B1 inhomogeneity at the base of the brain.
The MR protocols encompassed acquisitions using: ·
- Gradient Echo sequence (TE/TR= 3/44ms, FA 20°, Bw 290Hz/px, GRAPPA 4, FOV 192x192x96 mm3 for isotropic spatial resolutions of 0.5 and 0.3 mm and respective TA of 14 and 30 min) acquired in sagittal orientation; ·
- MPRAGE sequence (TI/TE/TR= 1100/2.7/2800ms, FA 4°, Bw 410Hz/px, GRAPPA 3, FOV 256x256x204 mm3 for isotropic spatial resolution of 0.8 mm); ·
- B0- and 8-transmit-channel B1-mapping from a 3D triple-echo Gradient Echo (GRE) and an interferometric pre-saturated 2D turbo-FLASH sequence (XFL) [4], respectively. These maps were used for kT-points pTx pulse design [5]; ·
- 2D CSI_STEAM sequence (TE/TM/TR=20/10/1000 ms, 7.5x7.5x10 mm3 nominal resolution).
Results
All monitored physiological parameters remained normal for the three monkeys during their MR examination. No change in their diet, weight and behavior were observed for a week after the exposure and each monkey was considered normal by the wellness manager and veterinarian.Figure 2 illustrates the quality of the 300-µm and 500-µm isotropic raw GRE and Maximum Intensity Projection images (MIP) of the same images. Figure 3 displays 800-µm T1-weighted MPRAGE images
Figure 4 presents the positioning of the MRSI slice and volume-of-interest for one of the monkeys. While the B0 homogeneity achieved across the voxel remained quite modest (FWHM ~80-90Hz), the local homogeneity was sufficient to obtain good quality spectra as shown by the spectral decomposition of one of them.
Figure 5 shows that, thanks to pTx kT-points [5], excitation homogeneity is reached in the entire brain, in particular in its lower part where the CP mode fails to bring up sufficient GRE signal. With 5 kT-points of 1.5-ms total duration, the NRMSE returned by our Bloch simulator was only 3% in the brain ROI for the 20°-target flip angle.
Discussion and Conclusion
No changes of any kind were observed in our monkeys following their “realistic” 11.7T MRI examination. This first step in assessing the innocuity of this unique medical device is positive confirming that magnetic field exposure for 90 minutes do not cause deleterious short-term effects on non-human primates.On the imaging side, the gradient-echo and stimulated echo based sequences used in pseudo-CP mode yielded satisfactory images and spectra. Classic PD and T1-weighted images of the monkey brain were acquired successfully without the need to rely on dedicated pulse design. Interestingly, GRE acquisitions exhibit very strong blood signal allowing for exciting high resolution MR brain angiography.
The B1 inhomogeneity observed using the pseudo-CP mode in the monkey brain was comparable to the one obtained with a volume coil at 3T on a human brain [6]. This is mostly due to the much smaller monkey brain volume (~100 cm3); still the adopted pTx kT-points approach was quite efficient suggesting that many sequence developments shall be feasible at 11.7T without the recourse to more powerful pTx approaches.
Because of our temporary technical constraints and the many challenges ahead, there is a lot of room for improvement upon this first work, whether for 1H MRSI or anatomical and functional MRI. More experiments will be carried out in monkeys in the next months with dedicated coils to tackle them.
Acknowledgements
This part of the Iseult project has been funded by CEA and BPIfrance. NB is funded by the European Union’s Horizon 2020 research and innovation program under grant agreement No 885876 (AROMA project).References
- D. Le Bihan, T. Schild, Human Brain MRI at 500MHz, scientific perspectives and technological challenges, Supercond. Sci. Techno. 2017;30:1-19.
- Luong M, et al, A Compact 16Tx-32Rx Geometrically Decoupled Phased Array for 11.7T MRI, ISMRM 2022, #707.
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672-9.
- Amadon A, et al, Validation of a very fast B1-mapping sequence for parallel transmission on a human brain at 7T, ISMRM 2012, #3358.
- Cloos M A, et al. KT-points: Short Three-Dimensional Tailored RF Pulses for Flip-Angle Homogenization over an Extended Volume, Magn Reson Med, 67(1):72-80, 2012.
- Boulant N, Le Bihan D, Amadon A. Strongly modulating pulses for counteracting RF inhomogeneity at high fields. Magn Reson Med. 2008 Sep;60(3):701-8.
Figures

From left to right, Iseult scanner in its archway, inside the
Iseult head coil the Iseult coil itself.
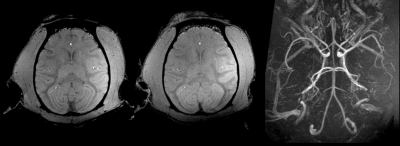
In
vivo MRI data acquired in one macaca rhesus at 11.7 T with 3D GRE sequences.
The two images on the left were registered and reconstructed in the transversal
plane with 3D multiplanar reformation (MPR) out of 500µm and 300µm isotropic
acquisitions. The image on the right side is an MIP made out of the 300µm
isotropic gre acquisition. Without any contrast agent enhancement, the images
shows a very detailed the animal brain arterial network.
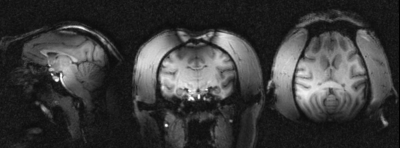
In
vivo MRI data acquired in one macaca rhesus at 11.7 T with 3D MPRAGE sequences
in pCP mode. The images were reconstructed from left to right respectively in
sagittal, coronal and transversal planes with 3D MPR out of 800um isotropic
acquisitions. The images reveals classic T1 weighted contrast but also strong
B0 artefact on the top of the head because of poor shimming and signal losses
mainly in the back of the head probably due to the inversion pulse operating out of
the adiabaticity regime.
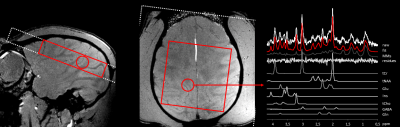
In
vivo 1H MRS data acquired in the macaca rhesus at 11.7 T (2D
CSI_STEAM, TE/TM/TR=20/10/1000 ms, sw 4 kHz, 2048 points, NA=12, weighted k-space
encoding scheme, in-plane resolution 7.5 x 7.5 mm2, slice thickness
10 mm). On the right panel, a typical spectral decomposition is shown using
LCModel 6.2 [3] and a dedicated basis set of model spectra.
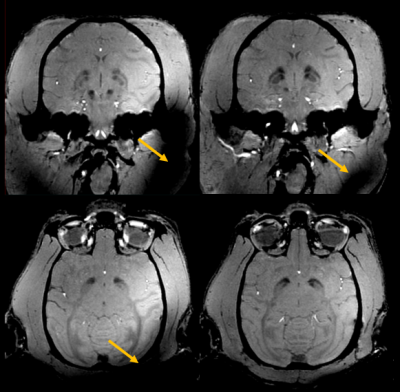
Pulse
design with parallel transmission on NHP. Comparison of GRE MRI stemming from
the default pCP mode (left) vs a dedicated kT-points pTx pulse
design (right). Both acquisitions are registered and reconstructed
in the coronal (top) and transversal planes (bottom) with 3D MPR out of 500µm
isotropic acquisitions. Orange arrows points at strong signal losses. They are more prominent on the pCP acquisition than on the pTx acquisition. One can notice that even with the very same image windowing, the contrast is sharper for the pTx acquisition, especially in the deep gray nuclei.
DOI: https://doi.org/10.58530/2023/3919