3915
MRI detection of gene expression in the mammalian brain using the Manganese transporter Zip141Laboratory of Functional and Molecular Imaging, NIH/NINDS, Bethesda, MD, United States
Synopsis
Keywords: Molecular Imaging, Cell Tracking & Reporter Genes, Brain, Neuroscience, Cell-type Imaging, Brain connectivity
We report the use of Zip14, a Manganese transporter, as an MRI-visible gene expression reporter system. Mice were intracranially injected into various brain areas with AAVs carrying a plasmid construct of Synapsin promoter for neuronal-specific expression, Zip14, and histological marker. MRI was performed immediately after, 2 weeks after, and 4 weeks after injection. Significant, large magnitude hyperintensity was observed 2 and 4 weeks after injection, but not immediately after, at the injection site and areas projecting away or towards the injection site. This gene expression reporter system is viable without the use of additional contrast agents or non-standard imaging protocols.Introduction
There has been considerable interest in developing genetically encoded reporters that alter MRI contrast for imaging in the mammalian brain. Many groups have exploited a variety of proteins to alter contrast mechanisms such as iron loading1, CEST agents2, transporters of Gd chelates3,4, and other methods5–7. Despite significant efforts, these techniques have been not widely adopted. This could be due to difficulty in contrast agent delivery to the brain and/or high overexpression requirements of exogenous proteins. We hypothesize that the metal transporter gene Slc39a14 coding for the protein Zip14 has potential as an MRI reporter system. Zip14 is highly expressed in the liver where it regulates blood Mn levels. Patients with loss-of-function mutations in Slc39a14 exhibit hyperintensity on T1w MRI of the brain due to increased blood Manganese (Mn) concentration8. Additionally, hyperintensity in Mn-enhanced MRI of the brain correlates with areas expressing higher levels of Zip149,10. Here we demonstrate that Zip14 overexpression in the brain using viral delivery produces long lasting, specific signal enhancement in vivo.Methods
The plasmid construct used in this study consisted of a Synapsin promoter for neuronal specific expression, Slc39a14 isoform 1, and a histology visible tag (either EGFP or FLAG) designed to be added to the C-terminus of Zip14. The plasmid was packaged into either AAV9 or retroAAV11 capsids for anterograde (AAV-Zip14) or retrograde (retroAAV-Zip14) labeling, respectively, with final working titers of 1x1012 vg/mL.Separate stereotaxic injections were performed on adult C57Bl6J mice at coordinates corresponding to the geometric centers of the S1 barrel cortex (S1BC), ventro posteromedial nucleus (VPM), or the caudate putamen (CP).
MRI was performed on a 11.7 T animal MRI system (Magnex Scientific) using a CryoProbe system (Bruker). Scan protocol included a 1 min low-resolution pilot and an 80 µm isotropic resolution MDEFT sequence [TE = 3.6 ms, TR = 4 s, TI = 1100 ms]. Imaging was performed at 0, 2, and 4 weeks post injection (±2 days).
After the last imaging session, animals were transcardially perfused with paraformaldehyde and cryoprotected in 30% sucrose for histology.
MRI were skull stripped using 3DPCNN12. Allen Mouse Brain Atlas (CCFv3) labels were registered to the image native space using ANTs. Further image analyses were performed using Python, MIPAV, and 3D Slicer. Statistical analyses were performed using R and GraphPad Prism.
Results
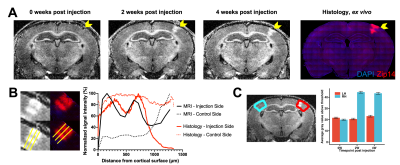
At 0 weeks post injection of AAV-Zip14-EGFP, no visually apparent differences in signal were observed in the S1BC. However, at 2 weeks post injection, there was marked hyperintensity in the S1BC with heterogeneity in intensity across the cortical layers. This signal enhancement pattern persisted through 4 weeks post injection. Immunohistochemistry validated that the MRI signal enhancement observed in the S1BC was due to Zip14 overexpression (Figure 1A). Well established neurotropism of AAV9 could explain the lack of MRI contrast and signal in immunohistology in layer 4 (Figure 1B). Comparison with matched histology revealed expression of Zip14 in the cell bodies and dendrites in the S1BC.MRI signal enhancement in the S1BC at each timepoint was quantified. After thresholding to the top 10% intensity voxels, no significant differences in signal were observed at 0 weeks post injection. Significant signal increases were observed the injection side S1BC compared to control at 2 and 4 weeks post injection (Figure 1C).
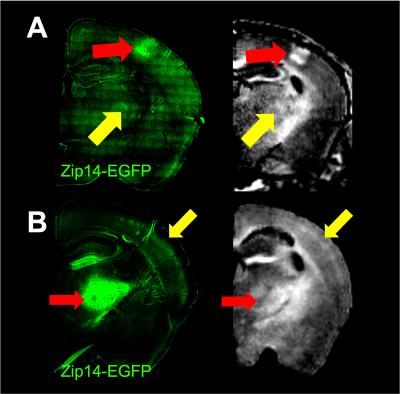
MRI enhancement could be detected in the processes of neurons that project from the S1BC to the thalamus. Histology confirmed Zip14-EGFP-positive processes in the thalamus, but not cell bodies. This indicates that the Zip14 moved anterograde down axons to the thalamus and caused MRI enhancement in the thalamus. Injections of AAV-Zip14-GFP into the VPM of the thalamus produced focal MRI signal enhancement in layer 4 of somatosensory areas of the cortex. Histology showed Zip14 overexpression in cell bodies and dendrites in the thalamus, and focal overexpression in the processes in cortical layer 4 as expected from anterograde transport from the injection site (Figure 2).
To determine if MRI enhancement could occur with retrograde viruses, retroAAV-Zip14-FLAG was injected into the CP. No significant MR signal enhancement at the injection site or posterior brain areas at 0 weeks post injection was detected. At 2 weeks post injection, focal hyperintensity was apparent at the injection site and several posterior areas. Subtracting the 2 week post injection from the 0 week post injection image and rendering in 3D allowed the sites of enhancement to be visualized at the injection site (CP) and areas projecting to the CP such as globus pallidus, pallidus, and substantia nigra (Figure 3).
Conclusion
Zip14 overexpression is an MRI-visible expression reporter system. The signal enhancement observed in our experiments was obtained without the use of additional contrast agents and without sophisticated image acquisition beyond vendor supplied sequences. The neural tracing application of AAV-Zip14 and retroAAV-Zip14 could be readily extended to other brain areas or used in the context of neurodevelopment, brain injury, or neurodegeneration models. It is not yet clear what the detection limit for Zip14 overexpression is. However, even at high levels of expression, modification of this construct to include other cell type-specific promoters or co-expression with other genes of interest should enable Zip14’s reporter applications to other cell types and organ systems.Acknowledgements
We thank Raymond Fields, Director of the NIH/NINDS/Viral Production Core Facility, for producing the viruses used in this study.
This research was supported by the intramural program at the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH).
References
- Deans, A. E. et al. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med 56, 51–59 (2006).
- Gilad, A. A. et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol 25, 217–219 (2007).
- Louie, A. Y. et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol 18, 321–325 (2000).
- Patrick, P. S. et al. Dual-modality gene reporter for in vivo imaging. Proc Natl Acad Sci U S A 111, 415–420 (2014).
- Koretsky, A. P., Brosnan, M. J., Chen, L., Chen, J. & van Dyke, T. NMR detection of creatine kinase expressed in liver of transgenic mice: determination of free ADP levels. Proceedings of the National Academy of Sciences 87, 3112–3116 (1990).
- Bartelle, B. B., Szulc, K. U., Suero-Abreu, G. A., Rodriguez, J. J. & Turnbull, D. H. Divalent metal transporter, DMT1: A novel MRI reporter protein. Magn Reson Med 70, 842–850 (2013).
- Mukherjee, A., Wu, D., Davis, H. C. & Shapiro, M. G. Non-invasive imaging using reporter genes altering cellular water permeability. Nat Commun 7, 1–9 (2016).
- Tuschl, K. et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat Commun 7, 11601 (2016).
- Rallapalli, H. et al. MEMRI‐based imaging pipeline for guiding preclinical studies in mouse models of sporadic medulloblastoma. Magn Reson Med 83, 214–227 (2020)
- Rallapalli, H., Darwin, B. C., Toro-Montoya, E., Lerch, J. P. & Turnbull, D. H. Longitudinal MEMRI analysis of brain phenotypes in a mouse model of Niemann-Pick Type C disease. Neuroimage 217, 116894 (2020).
- Tervo, D. G. R. et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92, 372–382 (2016).
- Chou, N., Wu, J., Bai Bingren, J., Qiu, A. & Chuang, K. H. Robust automatic rodent brain extraction using 3-D pulse-coupled neural networks (PCNN). IEEE Trans Image Process 20, 2554–2564 (2011).
Figures
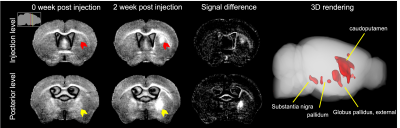
Zip14-retroAAV potentiates tracing parts of the cortico–basal ganglia–thalamic network. At 0 weeks post injection into the CP, no signal enhancement was apparent at the injection site (red arrow) or posterior sections (yellow arrow). At 2 weeks post injection, there was large magnitude signal enhancement apparent in the CP and posterior nuclei – a fact made clearer by longitudinal image subtraction. Insert – locations for the injection level (red) and posterior level (yellow) slices. Rendering the signal changes in 3D allows for complete visualization of the highlighted nuclei.