3906
Multi-channel, Stretchable, Self-Tuning Coil Array Based on Liquid Metal Technology
Elizaveta Motovilova1, Terry Ching2, Jana Vincent3, Ek Tsoon Tan4, Victor Taracila3, Michinao Hashimoto2, Fraser Robb3, Darryl Sneag4, and Simone Angela Winkler1
1Departmetn of Radiology, Weill Cornell Medicine, New York, NY, United States, 2Engineering Product Development Pillar, Singapore University of Technology and Design, Singapore, Singapore, 3GE Healthcare, Aurora, OH, United States, 4Department of Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States
1Departmetn of Radiology, Weill Cornell Medicine, New York, NY, United States, 2Engineering Product Development Pillar, Singapore University of Technology and Design, Singapore, Singapore, 3GE Healthcare, Aurora, OH, United States, 4Department of Radiology and Imaging, Hospital for Special Surgery, New York, NY, United States
Synopsis
Keywords: RF Arrays & Systems, RF Arrays & Systems, Liquid metal, Stretchable RF coils
Stretchable receive coils can provide conformal fitting, improved SNR, and dynamic imaging capabilities. However, conductor stretching alters the resonance frequency, reducing potential SNR advantages. Previously, we proposed and demonstrated in a single element prototype a smart self-tuning coil design which allows to maintain the desired Larmor frequency with stretching. This work investigates the applicability of self-tuning techniques in a multi-channel stretchable coil array and demonstrates its performance in silico and in vitro. Simulation results show stable resonance frequency (<1%) and sensitivity (±5%) for varied load/stretching conditions. In vitro imaging demonstrates consistent performance of individual coil elements and the array (SNR=146).Introduction
Stretchable liquid metal-based radiofrequency receive coils have demonstrated improved sensitivity, anatomic adaptability, and possibility for dynamic imaging1-5. However, conductor stretching alters the resonance frequency, reducing the SNR advantages of having a close-fitting receive coil. To mitigate inherent frequency shift with stretching, a smart coil design based on a self-tuning interdigital capacitor geometry was previously developed to maintain the desired Larmor frequency, and its feasibility was demonstrated via stretching of a single coil element design5. This work investigates the applicability of self-tuning techniques in a multi-channel stretchable coil array and demonstrates its performance in silico and in vitro.Methods
Simulations. To test and optimize the stretchable coil array design, full-wave numerical simulations were performed in COMSOL Multiphysics. The coil array comprised 6 elements, each containing a 7x6 cm rectangular loop with an integrated interdigital self-tuning capacitor5. The conducting traces were implemented as Gallium-filled (σ=7.1E6 S/m) microchannels of 0.5 mm diameter, embedded in a dielectric polymer (ε=2.7) substrate. The neighboring elements were decoupled using a critical overlap distance of 12 mm, which was previously determined from S21 simulations6. The 1x6 coil array was wrapped around a cylindrical homogeneous (ε=78, σ=0.46 S/m) phantom of length L=150 mm and variable diameter, D, to represent loading of different sizes, i.e. small (DS=109 mm), medium (DM=125 mm), and large (DL=142 mm). To load the coil array in its native (unstretched) state, the array was positioned circumferentially around a small cylinder. To simulate a degree of stretch of 15% and 30%, the medium and large cylinders, respectively, were used.Fabrication. The individual coil elements were fabricated using the direct ink writing (DIW) technique [7]. DragonSkinTM 30 silicone (Smooth-On) was spin-coated on a glass panel at 700 rpm for 40 s. Microchannel walls were printed on a DIW printer (SHOTmini200ΩX, Musashi, Japan) with fast-curing silicone sealant (SpeedSeal) used as a liquid ink. Liquid metal (GaIn) was injected into the microchannels. Copper wires were inserted at the terminals and connected to a printed circuit board containing tuning, matching, detuning, and preamplifier circuitry. The coil elements were arranged into a 1x6 array and attached using fast-curing silicone adhesive (SIL-poxy by Smooth-On) to form a cylindrical array of diameter D=125 mm. Figure 3 shows the fabricated coil array (a) positioned on a flat surface and (b) wrapped around the phantom.
In vitro imaging. Imaging experiments were performed on a 3T MRI system (MR750, GE Healthcare). A fast spin echo sequence with the following parameters was used: TR=3000 ms, ETL=16, FOV=24 cm, NEX=2, BW=±10.42 kHz, slice thickness=3 mm. A standard, homogeneous, cylindrical (L=150 mm, D=125 mm) phantom was used as a load.
Results
Simulations. Figure 1(a)-(c) show the 3D simulation model of the multi-channel coil array with the three differently sized load and stretching configurations. Figure 2(a) shows the simulated S11 parameters for the small (blue), medium (red), and large (yellow) cylinders. The simulation results demonstrate relative frequency stability with stretching and loading, with only 1 MHz (0.8%) maximum frequency variation (Figure 2(b)), which agrees well with our previously published single element stretching simulations5. Figure 2(c) shows the combined sensitivity (1 W-normalized B1- field magnitude) maps of all coil elements at different stretching/loading conditions. Although, the average magnetic field strength at the isocenter varies among the three phantoms as follows 0.92 uT±0.2 uT (±22%), the average magnetic field strength at the phantom surface remains relatively stable around 2.85 uT±0.15 uT (±5%) with stretching.In vitro imaging. Figure 4(a) shows the acquired individual sensitivity (SNR) maps of all coil elements, demonstrating consistent performance (<7% variation of SNR) when comparing individual elements. Figure 4(b) shows the combined SNR map of all coil elements, correlating well with the simulations, yielding an SNR of 146 at isocenter. Slight SNR differences in individual array elements are attributed to fabrication tolerances.
Conclusion
A multi-channel stretchable self-tuning coil array was studied in simulations and in vitro. Simulation results demonstrate that the resonance frequency was maintained within ±1 MHz (<1%) variation when the coil array was stretched from 0 to 30% by loading it with phantoms of different sizes ranging from diameters DS=109 mm to DL=142 mm. The measured sensitivity maps agreed well with those obtained from simulations, yielding an SNR=146 at isocenter, which demonstrates the feasibility of a multi-channel stretchable coil array based on the self-tuning liquid metal technology. Future work includes coil array optimizations and in vitro stretching tests.Acknowledgements
The authors acknowledge funding research support from the National Institutes of Health (R01 EB031820) and the invaluable support from GE Healthcare.References
- Varga, M., et al., Adsorbed eutectic GaIn structures on a neoprene foam for stretchable MRI coils. Advanced Materials, 2017. 29(44): p. 1703744.
- Mehmann, A., et al., Automatic resonance frequency retuning of stretchable liquid metal receive coil for magnetic resonance imaging. IEEE transactions on medical imaging, 2018. 38(6): p. 1420-1426.
- Mehmann, A., et al., On the bending and stretching of liquid metal receive coils for magnetic resonance imaging. IEEE Transactions on Biomedical Engineering, 2018. 66(6): p. 1542-1548.
- Port, A., et al., Detector clothes for MRi: A wearable array receiver based on liquid metal in elastic tubes. Scientific Reports, 2020. 10(1): p. 1-10.
- Motovilova, E., et al., Stretchable self-tuning MRI receive coils based on liquid metal technology (LiquiTune). Scientific reports, 2021. 11(1): p. 1-10.
- Motovilova, E., et al. Fabrication Methods for Stretchable, Self-tuning Multi-element Liquid Metal Coil Arrays (LiquiTune) in ISMRM. 2022. 7. Yamagishi, K., et al., Ultra‐Deformable and Tissue‐Adhesive Liquid Metal Antennas with High Wireless Powering Efficiency. Advanced Materials, 2021: p. 2008062.
Figures
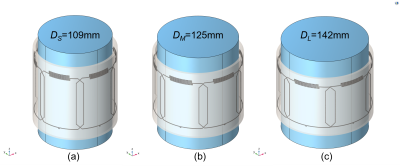
Figure 1. 3D
simulation models of the 6-channel coil array loaded with (a) small, (b)
medium, and (c) large phantoms.
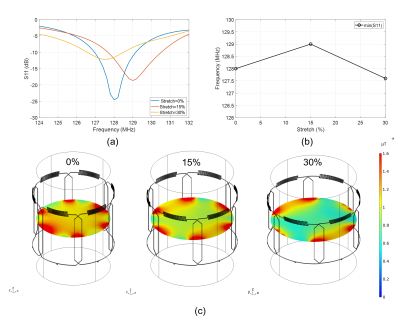
Figure 2. Simulation
results. (a) S11-parameter, (b) resonance frequency, and (c) 1W-normalized B1-
field magnitude changes with different
stretching/loading conditions.
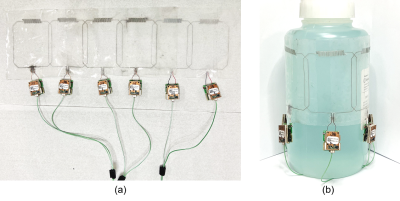
Figure 3. Fabricated
coil array when (a) flat and (b) loaded with a phantom.
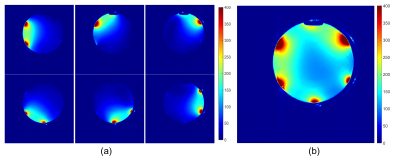
Figure 4. In vitro
MR imaging with the 6-channel stretchable coil array. (a) Individual and (b)
combined coil sensitivity maps.
DOI: https://doi.org/10.58530/2023/3906