3873
Deuterium oxide MRI to image the water inflow in the bovine lens of the eye
Xingzheng Pan1, Emily MacFarlane1, and Paul Donaldson1
1Physiology, University of Auckland, Auckland, New Zealand
1Physiology, University of Auckland, Auckland, New Zealand
Synopsis
Keywords: Deuterium, Aging, Human eye
In the absence of a blood supply the lens operates an internal microcirculation to deliver nutrients, remove metabolic wastes, and controll the lens volume, which together to maintain the optical properties of the lens. While this microcirculation is generated by circulating fluxes of ions and water, visualising water flow throughout the whole lens and especially into the central of lens in real time has proven challenging. To address this, we developed and optimised new deuterium oxide (D2O) MRI protocols to image water flow within multiple organ-cultured bovine lenses that can be routinely performed using 3T clinical MRI.Purpose
The lens is an avascular organ in the eye. It operates a microcirculation to uptake nutrients and export waste using water as a carrier1. As a negative imaging tracer, deuterium oxide (D2O) can track the water flow in real time with MRI2. In this study, we aim to establish and optimise protocols to visualise the regulation of water movement through the lens using deuterium oxide (D2O) contrast imaging.Methods
Lenses were extracted from bovine eyes obtained from a local abattoir, and up to 9 lenses were placed into a customised MRI-compatible sample holder (Fig.1) that contained isotonic artificial aqueous humour (AAH). At the start of the experiment (time = 0), the AAH bathing medium was replaced with either isotonic (290 mOsm/L), hypotonic (210 mOsm/L) or hypertonic (410 mOsm/L) D2O/AAH solutions to cause osmotic water fluxes or isotonic + ouabain (1mM) to inhibit the microcirculation3. Scans were performed by a 3T MRI (VIDA, Simens) equipped with a 16-channel hand/wrist coil. A proton-density (PD) - weighted image was acquired every 15 minutes using a turbo spin echo (TSE) sequence with FOV = 180×180 mm, matrix size = 512×512, TE = 8.70ms, TR = 2000ms, slice thickness = 3mm, and four averages. Scans were performed every 15 minutes for up to 2 hours to capture the change in signal intensity caused by the penetration of D2O into the lens under different conditions. To extract the rate of change in signal intensity, an ROI was drawn in the lens core to average the signal change in that region of the lens. The average signal of the core from different perturbations was normalised to its time 0 signal. The D2O flow rate into the lens core is calculated as the slope of the linear fitting of the normalised signal.Results
The usage of D2O in the bathing medium caused a progressive reduction in signal intensity as the D2O penetrated the lens and replaced H2O (Fig. 2A). This signal reduction was more apparent in the outer cortex of the lens, which has a higher water content than the central lens core, however, quantification of change in signal intensity in the core over time (Fig. 2B) showed that D2O did penetrate the lens core, as predicted by the microcirculation system at a rate of 1.70 ± 0.3 × 10-3 mins -1. Furthermore, this rate of delivery of D2O to the lens core could be increased (2.27 ± 0.3 × 10-3 mins-1) or decreased (1.3 ± 0.5× 10-3 mins -1) by exposing the lens to hypotonic or hypertonic stress respectively (Fig. 2C), while under isotonic conditions ouabain also decreased the delivery of D2O (1.5 ± 0.6 × 10-3 mins -1) to the lens.Discussion
Our use of D2O-MRI has shown that water fluxes are convected to the core of the lens as predicted by the microcirculation. The up-regulation of this microcirculation system has been proposed to be the target of developing novel anti-cataract therapies designed to enhance the delivery of anti-oxidants to the lens core, the lens region affected in age-related nuclear cataract1. Our new protocols thus offer the potential to identify and test therapies designed to enhance the delivery of such anti-cataract therapies.Acknowledgements
New Zealand Health Research Council Programme grant to fund this project.References
1. Donaldson, P. J., Grey, A. C., Maceo Heilman, B., Lim, J. C., & Vaghefi, E. (2017). The physiological optics of the lens. Prog Retin Eye Res, 56, e1-e24.
2. Vaghefi, E., Pontre, B. P., Jacobs, M. D., & Donaldson, P. J. (2011). Visualising ocular lens fluid dynamics using MRI: manipulation of steady state water content and water fluxes. Am J Physiol Regul Integr Comp Physiol, 301(2), R335-342.
3. Vaghefi E, Kim A, Donaldson PJ. Active maintenance of the gradient of refractive index is required to sustain the optical properties of the lens. Invest Ophthalmol Vis Sci. 2014;56:7195-7208.
Figures
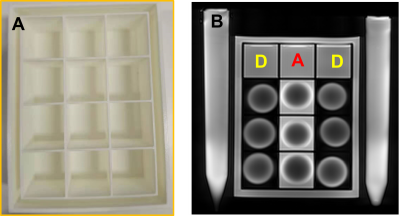
Customised sample
holder that allows high throughput of the lens being studied (A). A PD-weighted
image acquired with the TSE sequence shows two rows of the bovine lens in D2O
and one row of the lens in AAH (B). Tubes with water reference were imaged next
to the holder.
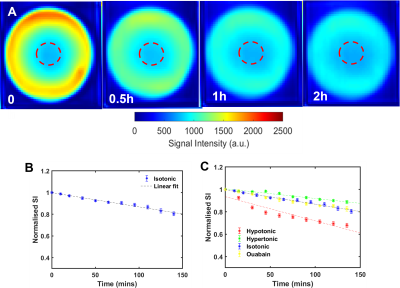
Time course of D2O penetration
into a bovine lens incubated in isotonic D2O/AAH (A). Averaged
normalised signal intensity obtained from the ROI (red circle) plotted against
time and fitted to extract a rate of delivery of D2O to the lens core (B). Effects of the decreasing (hypotonic) and increasing (hypertonic) extracellular osmolarity or inhibiting the
microcirculation (ouabain) on the rate of D2O penetration into the lens core (C) (N = 7 for each
group, mean ± std).
DOI: https://doi.org/10.58530/2023/3873