3864
In situ determination of pH in brain tissue using postmortem 1H-MRS1Institute for Biomedical Engineering, ETH Zürich, Zürich, Switzerland, 2Institute for Forensic Medicine, University of Zurich, Zürich, Switzerland, 3University Hospital for Psychiatry, University of Zurich, Zürich, Switzerland
Synopsis
Keywords: Spectroscopy, Spectroscopy
Postmortem, relatively low pH values have been described and reported to be dependent on the cause of death and the duration of the agonal phase. Using 1H-MRS on decedents and subsequent control pH measurements in collected samples, we investigated to what extent the pH dependence of peak positions of acetate and lactate are suitable to detect such low pH values non-invasively. We found that the measured chemical shift of acetate and corresponding pH value follow a simulated titration curve. Hence, acetate is a promising candidate to detect low postmortem brain pH for forensic investigations.Introduction
In forensic medicine, proton magnetic resonance spectroscopy (1H-MRS) of brain tissue has been conducted on decedents to study changes in postmortem interval or to diagnose fatal metabolic disorders non-invasively. In contrast to in vivo measurements, tissue temperature and pH can vary considerably postmortem. About ten hours after death, anaerobic glycolysis ceases at pH≈6.31, 2. However, previous studies have shown that lower postmortem brain pH values are possible, depending on the cause of death and the duration of the agonal phase3. Consequently, being able to determine this value non-invasively via in situ 1H-MRS might provide useful insights into the mechanisms of death. Previously, pH determinations via contrast agent-free 1H-MRS have been performed in brains using the downfield part of the spectrum at high field strength (9.4T)4. However, pH measurements based on chemical shift changes in the transition from acetate to acetic acid or lactate to lactic acid have not yet been attempted in brain tissue. These metabolites occur postmortem in measurable concentrations and are sensitive to pH changes in a meaningful range for postmortem studies. In our study, we investigate the feasibility of determining pH based from measured chemical shifts of acetate and lactate in decedents.Methods
1H-MRS measurements: As part of forensic judicial investigations, 23 decedents with planned autopsy were measured beforehand in a 3 T whole-body MRI scanner (Achieva, Philips Healthcare, Best, NL) using the body coil and an 8-channel phased-array receive-only head coil (Philips Healthcare, Best, NL). Single-voxel 1H-MRS measurements were performed in white matter tissue in the right hemisphere with a voxel volume of 6 ml and 256 signal averages (PRESS localization, TE/TR: 26/2288 ms, VAPOR water suppression, second order shimming, volume based RF power optimization). The chemical shifts in the recorded spectra were determined using peak picking in MATLAB.pH calculations based on chemical shifts: To calculate pH based on chemical shifts, myo-inositol was chosen as reference metabolite based on its pKa value and its chemical structure. Myo-inositol, acetate and lactate methyl doublet positions were identified via peak picking. An adapted Henderson-Hasselbalch equation was used to calculate pH5, 6:
$$(1): pH_{MRS}=pK_A+log_{10}{\frac{(δ_{obs}-δ_{acid})}{(δ_{base}-δ_{obs} )}}$$
In this equation, $$$pK_A$$$ of the indicator species lactate or acetate is used. $$$δ_{obs}$$$ is defined as the measured difference between lactate doublet or acetate and myo-inositol peaks. $$$δ_{acid}$$$ and $$$δ_{base}$$$ are adapted acidic and basic limits for these chemical shift differences6, 7 (Table 1).
Verification measurements: During autopsy of the 23 decedents, a brain sample of mainly white matter was taken. The samples were homogenized using a mortar. Then, tissue pH was measured using a pH-meter (pH-meter 761 Calimatic, Knick, Berlin, DE) in combination with a thermometer (HD 2307.0 RTD thermometer). To investigate the behaviour of the lactate doublet and acetate peak depending on pH, the observed chemical shifts were plotted against the measured pH values and compared to a simulated titration curve based on equation (1) and the values in Table 1.
Results
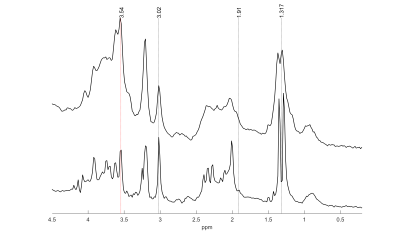
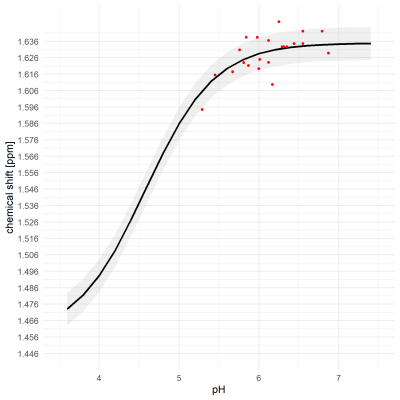
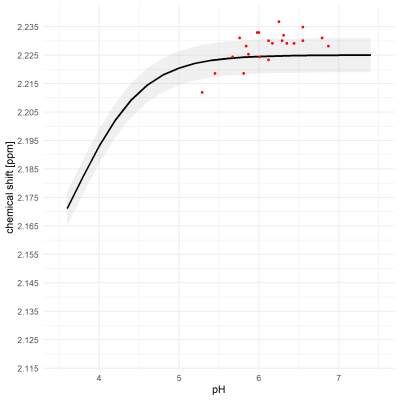
Exemplary MR spectra are shown in Figure 1.The median full-width-at-half-maximum (FWHM) of the water peak was 7.6 (min: 4.4, max: 9.3). Median SNR of lactate methyl doublet was 144.6 (min.: 70.0, max.: 166.3).The pH values measured in the samples ranged from 5.29 to 6.79, with 15 of 23 below pH = 6.3. $$$δ_{obs}^{acetate}$$$ and $$$δ_{obs}^{lactate}$$$ are shown in Figure 2 and Figure 3, respectively. The measured $$$δ_{obs}^{acetate}$$$ values closely follow the simulated titration curve based on equation (1) and the values in Table 1 (Figure 2). This is not the case for $$$δ_{obs}^{lactate}$$$ (Figure 3).
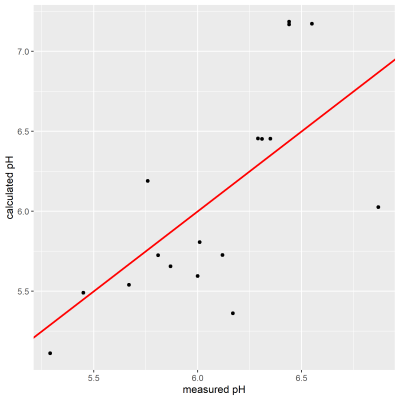
Finally, pH calculations were performed using $$$δ_{obs}^{acetate}$$$. Comparing $$$pH_{MRS}$$$ to the measured values, it was seen that they matched for pH < 6.3 (Figure 4). However, in two cases acetate shift was higher than $$$δ_{base}^{acetate}$$$ leaving $$$pH_{MRS}$$$ undefined, despite low pH in the corresponding sample.
Discussion
Despite the small study population, this work confirms that brain tissue pH goes clearly below 6.3 and that these low pH values are generally well reflected in the chemical shift changes observed for acetate.Peak picking for the position determination of acetate was challenging in some cases due to partial overlap with the N-acetylaspartate peak. This can potentially be improved by using fitting approaches, e.g. LCModel9. pH calculations could be further improved by evaluating the temperature and ionic strength dependence of equation (1).
The position of Lactate was detected reliably postmortem even if simple peak picking was used. However, in this study so far only few pH values were measured, where the chemical shift of lactate was expected to change. According to the first results, the observed chemical shifts do not match the expectations as indicated in Figure 3. More measurements are necessary.
As 15 out of 23 measured pH values were below 6.3, and postmortem brain pH was reported to depend on antemortem events3, determination of brain pH using 1H-MRS might provide useful information for case clarification during noninvasive examination of decedents.
Conclusion
In postmortem brains, pH below the expected value of 6.3 were observed. Such low in situ pH values are reflected in changed chemical shifts of lactate doublet and acetate. Calculation of pH based on chemical shifts is promising for acetate, but yet fails for the lactate doublet.Acknowledgements
No acknowledgement found.References
1. Madea BBB. Handbuch gerichtliche Medizin Band 1. 1 ed. Heidelberg: Springer-Verlag Berlin 2004.
2. Lang F. Säure-Basen-Haushalt. In: Ralf Brandes FL, Robert F. Schmidt, editor. Physiologie des Menschen. 32: Springer Berlin, Heidelberg; 2019. p. 457-68.
3. Monoranu CM, Apfelbacher M, Grünblatt E, Puppe B, Alafuzoff I, Ferrer I, et al. pH measurement as quality control on human post mortem brain tissue: a study of the BrainNet Europe consortium. Neuropathology and applied neurobiology. 2009;35(3):329-37.
4. Borbath T, Murali-Manohar S, Wright AM, Henning A. In vivo characterization of downfield peaks at 9.4 T: T(2) relaxation times, quantification, pH estimation, and assignments. Magn Reson Med. 2021;85(2):587-600.
5. Constantinides C. Spectroscopy and Spectroscopic Imaging. Magnetic Resonance Imaging: the basics: CRC Press; 2014.
6. Ackerman JJ, Soto GE, Spees WM, Zhu Z, Evelhoch JL. The NMR chemical shift pH measurement revisited: analysis of error and modeling of a pH dependent reference. Magnetic resonance in medicine. 1996;36(5):674-83.
7. Tredwell GD, Bundy JG, De Iorio M, Ebbels TM. Modelling the acid/base (1)H NMR chemical shift limits of metabolites in human urine. Metabolomics : Official journal of the Metabolomic Society. 2016;12(10):152.
8. Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265(2):54-84.
9. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in biomedicine. 2001;14(4):260-4.Figures