3863
Altered cortical metabolism contributes to cognitive deficits in a healthy military cohort
Julie M Joyce1,2, Kristin Heaton3, Huijun J Liao2, and Alexander P Lin2
1cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, Ludwig-Maximilians-Universität, Munich, Germany, 2Center for Clinical Spectroscopy, Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 3Military Performance Division, United States Army Research Institute of Environmental Medicine, Natick, MA, United States
1cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, Ludwig-Maximilians-Universität, Munich, Germany, 2Center for Clinical Spectroscopy, Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States, 3Military Performance Division, United States Army Research Institute of Environmental Medicine, Natick, MA, United States
Synopsis
Keywords: Spectroscopy, Neuro, Military
The selection of control groups is of increasing interest in military research. Here, we used single voxel magnetic resonance spectroscopy (MRS) to probe cortical metabolism in the anterior and posterior cingulate gyri, temporal lobe and parietal white matter in healthy military service members and civilian controls. Computerized neuropsychological tests were administered to evaluate neurocognitive abilities. We identified higher incidence of cognitive deficits in military service members relative to civilian controls as well as associations between neurometabolite levels and poor cognitive performance.Introduction
Military personnel who serve in combat are at elevated risk for post-traumatic stress disorder (PTSD) and mild traumatic brain injury (mTBI).1 These conditions share overlapping symptomology, including cognitive difficulties.2 A few studies have used civilian control groups to investigate the impact of PTSD and mTBI on brain structure, function and metabolism.3-5 However, the use of civilian control groups does not account for exposure to subconcussive blast injuries or the nonspecific effects of psychological stress incurred through time in combat.Previous work has shown that neurometabolite alterations exist between healthy military and civilian controls.6 Furthermore, it is known that subconcussive blast injuries and psychological stress can contribute to poor performance on neuropsychological tests.7,8 It has yet to be determined whether alterations in cortical metabolism contribute to these cognitive deficits.
The primary aim of this study was to use magnetic resonance spectroscopy (MRS) to examine associations between neurometabolite levels and neurocognitive abilities across healthy U.S. military service members and civilian controls. We examined five neurometabolites critical for brain health tNAA (N-acetyl-aspartate+N-Acetyl aspartyl glutamate), tCr (creatine+phosphocreatine), tCho (phosphorylcholine+glycerophosphocholine), Glx (glutamate+glutamine) and mI (myo-inositol) to gain insight into cortical metabolism in military service members. Secondly, we sought to characterize incidence of cognitive deficits in military and civilian groups.
Methods
Civilians and military service members with no self-reported history of neurological disorders, psychological disorders or drug addiction were recruited for this study. Neurocognitive abilities and mood were assessed using the Automated Neuropsychological Assessment Metrics (ANAM) battery.MRI scans were performed on a 3T Siemens MAGNETOM Skyra scanner using a 32-channel head coil. MRS data were acquired in four brain regions as shown in Figure 1: anterior cingulate gyrus (20x20x20mm), posterior cingulate gyrus (20x20x20mm), left temporal cortex (20x15x15mm) and parietal white matter (20x20x20mm). MRS data were acquired with the following parameters: point-resolved spectroscopy (PRESS), TR/TE=2000/30ms, 128 water-suppressed averages, 16 unsuppressed averages, bandwidth=1200Hz, 1024 data points. Neurometabolite levels were expressed as ratios to the unsuppressed water signal.
The MRS pre-processing pipeline included channel combination using singular value decomposition (SVD), spectral registration (for frequency drift) and eddy current correction. All data were analyzed in LCModel.9 Neurometabolite values with Cramer Rao Lower Bounds (CRLB) greater than 20% and extreme outliers [>3*interquartile range (IQR) above 3rd quartile or below the 1st quartile] were excluded from analyses.
ANAM performance was evaluated by examining rates of poor performance, below established cut-offs10 (<10th percentile of normative scores, adjusted for age, education and sex). Independent samples t-tests were used to assess differences in neurometabolite levels between groups in each brain region to compare with previous results.6 Multiple linear regressions were conducted to evaluate the associations between cognitive scores and neurometabolite levels (tNAA, tCho, tCr, Glx or mI) as well as other relevant factors [group (civilian, military), age and years of education].
Results
Sixty participants (21 civilian, 39 military) aged 19 to 53 years completed a MRS scan and the ANAM battery. Demographics, clinical questionnaire scores (PTSD Checklist-5, Beck Depression Inventory) and results of the ANAM mood scale are summarized in Table 1.Glx in the posterior cingulate (t= -2.97, p=0.004, d= -0.82) and parietal white matter (t= -2.12, p= 0.019, d= -0.59) were significantly lower in the military service members relative to civilians (Figure 3). Incidence of poor performance on ANAM cognitive tests was 3-28% higher in military service members relative to the civilian group (Figure 2).
In the anterior cingulate, regression models including tNAA (F=3.849, p=0.009, R2=0.251), tCho (F=4.195, p=0.005, R2=0.259), tCr (F=5.281, p=0.001, R2=0.289), Glx (F=2.937, p=0.031, R2=0.211) and mI (F=5.001, p=0.002, R2=0.282) significantly predicted ANAM “Matching to Sample” (M2S) scores. Parietal white matter tCho was also a significant predictor of M2S scores (F=3.162, p=0.021, R2=0.193) (Table 2). No other regression models found neurometabolite levels to be associated with cognitive results.
Discussion
Despite similar age and sex distributions, military service members exhibited lower levels of posterior cingulate and parietal white matter Glx than civilian controls. This finding is consistent with earlier comparison of neurometabolite levels in a smaller subset of this cohort.6 We have extended these findings through evaluation of a comprehensive neuropsychological battery and found higher incidence of deficits across multiple cognitive domains in the military group.The greatest differences in cognitive performance between groups was found on the ANAM (M2S) test which assesses spatial processing and visuospatial working memory. Interestingly, all significant neurometabolite associations with cognitive performance involved M2S, with higher metabolite levels predicting better cognitive scores.
It is important to note that tissue atrophy may affect neurometabolite levels. However, previous studies have identified neurometabolite alterations in individuals with mTBI and PTSD in the absence of tissue atrophy.11,12 In future analyses, we will apply a tissue correction to investigate whether tissue differences impact our MRS results.
Conclusion
We conclude that altered cortical metabolism is associated with poor visuospatial performance and may contribute to higher incidence of cognitive deficits in military service members relative to civilian controls. These findings emphasize the importance of control group selection in military research.Acknowledgements
This study was funded by DOD CDMRP WX81-XWH-10-1-0835. The views expressed in this abstract are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government.References
- Combs HL, Berry DT, Pape T, et al. The effects of mild traumatic brain injury, post-traumatic stress disorder, and combined mild traumatic brain injury/post-traumatic stress disorder on returning veterans. J Neurotrauma. 2015;32(13):956-966. doi:10.1089/neu.2014.3585
- Bryant R. Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):251-262. doi:10.31887/DCNS.2011.13.2/rbryant
- de Lanerolle NC, Hamid H, Kulas J, et al. Concussive brain injury from explosive blast. Ann Clin Transl Neurol. 2014;1(9):692-702. doi:10.1002/acn3.98
- Cartwright PE, Perkins TG, Wilson SH, Weaver LK, Orrison WW. Analysis of magnetic resonance spectroscopy relative metabolite ratios in mild traumatic brain injury and normative controls. Undersea Hyperb Med. 2019;46(3):291-297.
- Peskind ER, Petrie EC, Cross DJ, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage. 2011;54(Suppl 1):S76-S82. doi:10.1016/j.neuroimage.2010.04.008
- Liao HJ, Heaton K, Merugumala P, Saurman J, Orlovsky I, Merugumala SK, Rudolph K, Murphy N, Rowland B, Lin AP. Reduced NAA and glutamate in healthy military subjects compared to civilian controls. Proc Int Soc Magn Reson Med. 2015.
- Haran FJ, Handy JD, Servatius RJ, Rhea CK, Tsao JW. Acute neurocognitive deficits in active duty service members following subconcussive blast exposure. Appl Neuropsychol Adult. 2021;28(3):297-309. doi:10.1080/23279095.2019.1630627
- van Wingen GA, Geuze E, Caan MW, et al. Persistent and reversible consequences of combat stress on the mesofrontal circuit and cognition. Proc Natl Acad Sci U S A. 2012;109(38):15508-15513. doi:10.1073/pnas.1206330109
- Provencher SW. Estimation of Metabolite Concentrations from Localized in Vivo Proton NMR Spectra. Magn Reson Med. 1993; 30:672-679.
- Meyers JE & Vincent AS. Automated Neuropsychological Assessment Metrics (v4) military battery: Military normative data. Military Medicine. 2020;185(9-10):e1706–e1721. doi:10.1093/milmed/usaa066
- Wang W, Sun H, Su X, et al. Increased right amygdala metabolite concentrations in the absence of atrophy in children and adolescents with PTSD. Eur Child Adolesc Psychiatry. 2019;28(6):807-817. doi:10.1007/s00787-018-1241-x
- Kubas B, Lebkowski W, Lebkowska U, Kułak W, Tarasow E, Walecki J. Proton MR spectroscopy in mild traumatic brain injury. Pol J Radiol. 2010;75(4):7-10.
Figures
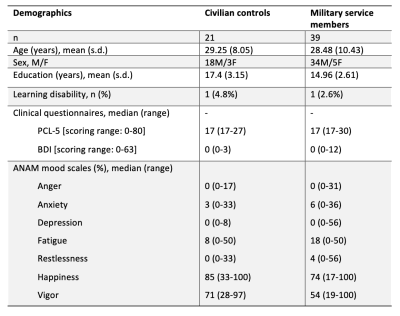
Table 1. Demographic characteristics of participants including age (years), sex (proportion males: females), education (years) and learning disability (%) across civilian and military groups. Median scores for PCL-5, BDI and ANAM mood scale are reported with scoring ranges provided for reference. PCL-5: PTSD Checklist-5; BDI: Beck Depression Inventory. ANAM: Automated Neuropsychological Assessment Metrics.
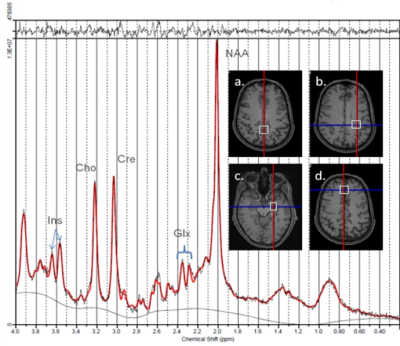
Figure 1. An example of LCModel processed spectrum acquired from parietal white matter in a healthy military subject. Inset: the four different MRS voxel locations studied: a. posterior cingulate gyrus, b. parietal white matter c. left temporal cortex, d. anterior cingulate gyrus.
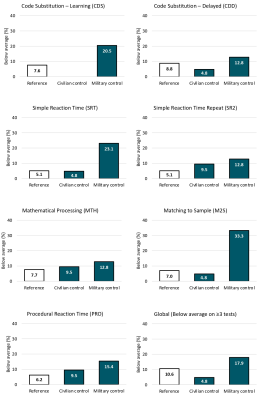
Figure 2. ANAM cognitive results in civilian and military groups. Throughput scores (number of correct responses per minute of available response time) are considered a measure of effectiveness or cognitive efficiency and were used to assess ANAM performance. Scoring accounted for age, sex and education. “Below average” performance is defined as <10th percentile using previously established cutoffs. Reference data (white) was obtained from Meyers et al. 2020.
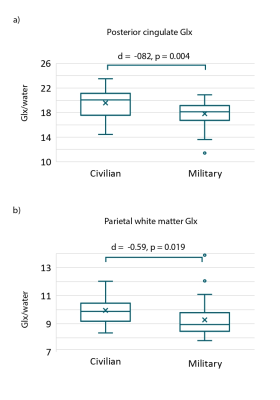
Figure 3. Boxplots depicting Glx levels in military and civilian groups in the posterior cingulate and parietal white matter. Significantly lower levels of Glx were identified in the military group in both regions. Glx levels are expressed as ratios to the unsuppressed water signal.
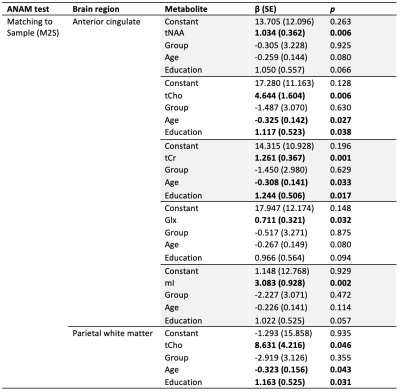
Table 2. Multiple linear regression results examining metabolites (tNAA, tCho, tCr, Glx or mI), group (military, civilian), age and years of education as predictors of ANAM neurocognitive scores. ANAM throughput scores (number of correct responses per minute of available response time) were used as the dependent variable in analyses. Only regression models where metabolites were a significant predictor are shown. Significant predictors are bolded.
DOI: https://doi.org/10.58530/2023/3863