3852
Toward vessel-suppressed cerebrovascular reactivity (CVR) mapping by using crusher gradients1Department of Radiology, Johns Hopkins University, Baltimore, MD, United States
Synopsis
Keywords: fMRI, fMRI
Cerebrovascular reactivity (CVR) is an indicator of cerebrovascular reserve and provides important information about vascular health in a range of brain conditions and diseases. However, current CVR maps often have artifactual bright spots in arterial/venous areas due to the complexity of the BOLD signal mechanism. In this work, we proposed a novel CVR acquisition scheme by adding single-direction or varying-direction crusher gradients to the BOLD sequence. The results demonstrated that these modified sequences could successfully suppress large-vessel artifacts in CVR map.
Introduction
Cerebrovascular reactivity (CVR) denotes the ability of cerebral vessels to dilate in response to vasoactive challenges, and is a measure of cerebrovascular reserve.1 This physiological parameter is thought to be an important index of the brain’s vascular health and has found applications in both large-vessel disease (e.g., arterial stenosis, stroke) and small-vessel disease (e.g., vascular dementia).2 CVR is typically measured with CO2-inhalation while BOLD MRI images are collected continuously. However, because of the complexity of the BOLD signal mechanism, CVR maps obtained with the conventional BOLD sequence often contain hyperintensive spots in regions rich in large arteries (due to inflow effect) or veins (due to venous cerebral blood volume effects). These bright spots are artifactual and could dilute the sensitivity of CVR to disease. Therefore, in this work, we proposed two modified BOLD sequences by adding bipolar crusher gradients before the acquisition module in an effort to suppress arterial/venous hyperintensive signals in the CVR map.Methods
Pulse SequenceWe compared CVR mapping results using three different BOLD sequences (Figure 1a): 1) The conventional BOLD MRI sequence; 2) Single-direction-gradient BOLD (SG-BOLD). We added a bipolar crusher gradient before the EPI acquisition module in BOLD along the foot-head direction; 3) Varying-direction-gradient BOLD (VG-BOLD). The sequence is similar to the one used in #2 but we varied the direction of the bipolar gradient across different dynamics (measurements) of the BOLD image series. A total of six different directions were randomized throughout the 200 BOLD dynamics. The variation pattern of the gradient direction was confirmed to be uncorrelated with the CO2-inhalation timing/paradigm. The design of Sequence #2 is to suppress arterial/venous signals so that they will not contribute to the CO2-induced signal change. However, not all vessels flow along the foot-head direction. Therefore, the design of Sequence #3 is to randomly vary the gradient direction across dynamics, thus vessels with any orientation will experience an elevation in “noise”, which will reduce the correlation between the CO2 and BOLD time-courses. Other imaging parameters were identical among the three sequences: single-shot gradient-echo EPI, field-of-view (FOV)=220×220×110mm3, voxel size=3.44×3.44×3.8mm3, TR/TE/flip angle=1500 ms/36 ms/64°, 26 slices, velocity-encoding=0.4 cm/s (if applicable), scan duration=5 min.
MRI experiment
Three healthy volunteers (2 males and 1 female, aged 22.7±2.1 years) were enrolled. All scans were performed on a Philips 3T system. Each subject underwent a scan session illustrated in Figure 1b. The three CVR-BOLD runs used the three sequences described above, in a randomized order across subjects. The CO2-inhalation procedure followed the method described in Lu et al.3 Briefly, the subjects were fitted with a nose clip, and breathed room air and hypercapnic gas (fixed inspiration of 5% CO2, 21% O2 and 74% N2) in an interleaved fashion (Figure 1c, 50s CO2, 70s room air, repeated twice) through a mouthpiece. End-tidal CO2 (EtCO2), the CO2 concentration in the lung which approximates that in the arterial blood, was recorded using a capnograph device.
Data processing
CO2-inhalation CVR data were processed using procedures detailed previously.3 Briefly, after pre-processing of BOLD image series, a general linear regression was performed in which the BOLD time-course was used as the dependent variable and the EtCO2 time-course was the independent variable, with a linear term as covariate to account for hardware-heating-related signal drift. This analysis was conducted on a voxel-by-voxel basis. This analysis yielded the T score map, which represents the statistical significance of the CVR estimation of each voxel.
Additionally, a coefficient-of-variation (CoV) map was computed from the first 20 dynamics for each run. This allows us to evaluate the expected augmentation of noise level in vessel but not in tissue voxels, before any CO2 effects are present.
Results and Discussion
Figure 2 shows the averaged CoV maps of three different pulse sequences. Consistent with our expectation, upon adding the crusher gradients, CoV (i.e., noise level) in the vessel-rich regions (arrows in Figure 2) was selectively elevated, especially when using the varying-direction-gradient sequence. In contrast, CoV in the tissue regions was relatively unchanged. Region-of-interest (ROI) results are shown in Figure 3. Compared to conventional BOLD sequence, CoV in the vessel regions (Figure 3a) increased by 195% and 264% in the SG-BOLD and VG-BOLD sequences, respectively. The CoV values in the tissue regions (Figure 3b) also increased (presumably due to diffusion-related signal attenuation), but only by 21% and 35%, respectively. Importantly, the tissue CoV values consistently remained below 2%.Figure 4a shows the comparison of T-score maps of different pulse sequences from a representative subject. We can observe that the bright artifacts from the conventional BOLD sequence are reduced by adding a single direction gradient, and are further reduced with the varying-direction gradients. A further ROI analysis (Figure 4b) reveals that the SG-BOLD and VG-BOLD sequences decrease the T-score value in vessel regions by 44% and 58% respectively.
Conclusions
In this study, we proposed a novel CVR acquisition scheme by adding single-direction or varying-direction crusher gradients to the BOLD sequence. Our results demonstrated that the addition of crusher gradient can successfully suppress large-vessel artifacts in CVR map.Acknowledgements
No acknowledgement found.References
1. Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. Neuroimage 2019;187:104-115.
2. Liu P, Xu C, Lin Z, Sur S, Li Y, Yasar S, Rosenberg P, Albert M, Lu H. Cerebrovascular reactivity mapping using intermittent breath modulation. Neuroimage 2020;215:116787.
3. Lu H, Liu P, Yezhuvath U, Cheng Y, Marshall O, Ge Y. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J Vis Exp 2014. doi: 10.3791/52306.
Figures
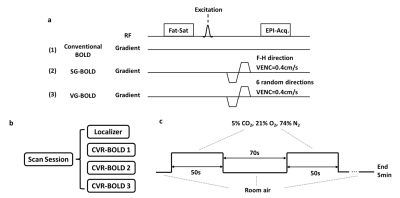
Figure 1. Illustration of the experimental design. (a) Three CVR-BOLD pulse sequence diagrams implemented in the scan. (b) Each subject went through three CVR-BOLD scans, each implementing a different pulse sequence. (c) The CO2-inhalation paradigm during each scan.

Figure 2. Coefficient of variation (CoV) maps of three different pulse sequences from the group average (N=3).
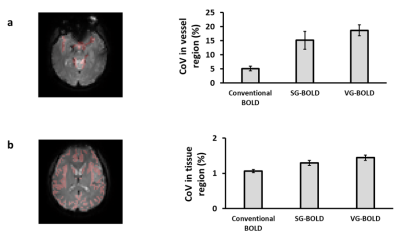
Figure 3. Comparison of CoV values in ROIs. (a) Vascular ROI in a representative subject and the mean CoV values in vascular regions (N=3). (b) Tissue ROI in a representative subject and the mean CoV values in tissue regions (N=3).
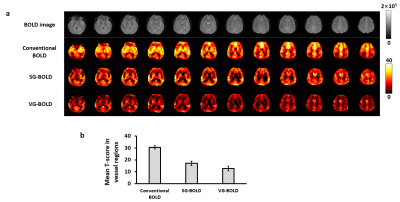
Figure 4. (a) Comparison of T-score maps of different pulse sequences from one representative subject. (b) ROI analysis of mean T-score value in vessel regions.