3829
Association between angioarchitecture and flow parameters in ruptured and unruptured arteriovenous malformation: a pilot study by 4D flow MR1Radiology, Sichuan Provincial People's Hospital, Chengdu, China, 2Center for Biomedical Imaging Research Beijing, Qinghua University, Beijing, China, 3Neurosurgery, Sichuan Provincial People's Hospital, Chengdu, China, 4Siemens Healthineers Ltd., Shanghai, China
Synopsis
Keywords: Flow, Velocity & Flow, arteriovenous malformation
The hemodynamics of cerebral AVM likely vary with lesion angioarchitecture and the rupture status, which couldn’t be reflected by morphology-based imaging. In this pilot study, 9 patients with AVM had their complicated feeding/draining patterns visualized by 4D-flow MR. AVM tend to have heterogeneous hemodynamics even with the same Spetzler-Martin grade or similar angioarchitecture. It tends to have smaller flow of the feeding artery after rupture. AVM with deep vein drainage tends to have diffused nidus and higher wall shear stress adjacent to the nidus. Higher flow of the feeding artery was associated with higher dynamic pressure and larger nidus volume.Introduction
The hemodynamics of cerebral arteriovenous malformation (AVM) could hardly be reflected by conventional morphology-based imaging and clinical grading. The relationship between lesion angioarchitecture (which closely relates to its pathophysiology), hemodynamics, and ruptured status have not been fully explored. The objective was to investigate the association between the angioarchitecture and flow-derived parameters by 4D flow MR imaging in both ruptured and unruptured cerebral AVM.Methods
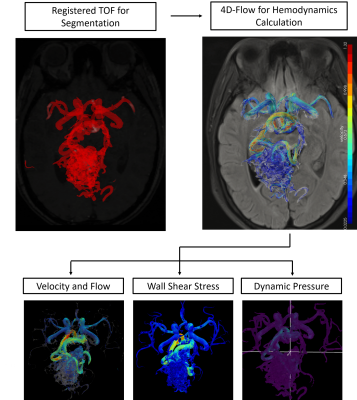
Consecutive patients with DSA diagnosed cerebral AVM, which was either unruptured, or ruptured with clinical Hunt-Hess grade I or II were prospectively enrolled. TOF-MRA was performed to assess the lesion structure. Information regarding lesion angioarchitecture were compensated by the DSA results. Spetzler-Martin grade of the lesion were recorded. 4D flow MR, a time-resolved RF-spoiled gradient echo sequence with short TR encoded by three spatial directions, was performed to assess the lesion hemodynamics, with technical details: a 3T scanner (Siemens VIDA, Germany); VENC= 120 cm/s; voxel size = 1.0mm isotropic. Prospective ECG gating and respiration-controlled navigator was used to perform scanning under free-breathing. A workflow reported by Fu. et al1 was used to obtain quantified values. The workframe is shown in Figure 1. The 3D model of AVM was generated by threshold segmentation of registered TOF imaging. 3D velocity filed calculated from 4D flow MRI was used for calculating hemodynamic parameters. For advanced hemodynamic parameters,we calculated wall shear stress(WSS) of the feeding artery adjacent to the nidus, dynamic pressure(DP), pulsatility Index(PI), resistant index(RI) by following equations: $$WSS=μ(\frac{\partial v_{parallel}}{\partial y})_{y=0}$$ $$DP=\frac{1}{2}\rho v^2$$ $$PI = (peak systolic velocity - minimal diastolic velocity) / (mean velocity) $$ $$RI = (peak systolic velocity - minimal diastolic velocity) / (peak velocity) $$ The time-averaged WSS and DP were calculated using the average of obtained value at all timepoints during a cardiac cycle. Flow-derived parameters were compared between groups with different angioarchitectures and rupture status. Correlations between flow and multiple flow-derived advanced parameters were examined.Results
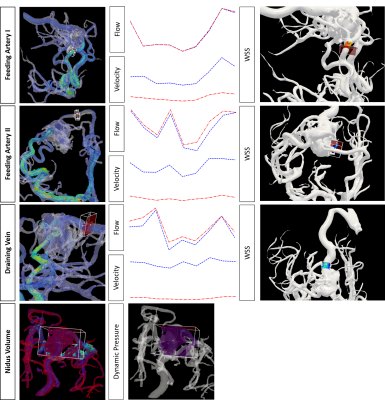
Eleven patients were firstly enrolled, and 2 patients were excluded (one with ruptured AVM, and the lesion was completely obscured by the hematoma on MR; one with a hypointensity nidus after radiotherapy, which precluded accurate segmentation). Nine patients (4 male and 5 female, age range:19-58 years old, 4 with unruptured and 5 with ruptured AVM) finished the evaluation. In terms of angioarchitecture, there were no significant association between rupture status and Spetzler-Martin grade (p=0.88). Feeding by multiple arteries was significantly associated with unruptured status (p<0.001, r=0.949). Deep vein drainage was significantly associated with diffusely distributed nidus (compared with compact nidus, p=0.011), and also significantly associated with higher WSS adjacent to the nidus (3.13 vs. 1.71, p=0.033). In terms of flow assessment, the total flow ranged from 4.38 to 8.34 ml/s among the 4 cases with Spetzler-Martin grade II, and ranged from 4.03 to 15.34 ml/s among the 3 cases with Spetzler-Martin grade III, which showed substantial overlap. The mean velocity of the feeding artery was 0.54±0.23 cm/s in unruptured group and 0.21±0.21 cm/s in the ruptured group (p=0.397); the total flow was 8.02±5.01 ml/s in unruptured group and 2.88±3.50 ml/s in the ruptured group (p=0.111). There were no significant differences in the velocity or flow of the feeding artery between AVM with deep and superficial vein drainage, or between AVM with or without aneurysms within the nidus. Among the flow-derived parameters, mean and maximal flow of the feeding artery were highly correlated with DP (both p=0.037, r=0.9), and also with nidus volume (p=0.037, r=0.9). PI and RI were highly correlated (p<0.001). Outflow ratio (venous outflow/nidus volume) was highly correlated with mean flow, maximal flow of the feeding artery, and WSS (all p<0.001).Discussion
The hemodynamics of AVM are believed to contribute to its pathophysiology and clinical presentation2. Nevertheless, the hemodynamics of AVM are complex and likely vary with lesion angioarchitecture and the rupture status3. In this pilot study, successful intracranial flow evaluation by 4D flow MR was achieved in 9 out of 11 patients. This technique makes it possible to measure vector blood flow with high temporal resolution in three anatomical dimensions4. Image quality which was sufficient for the following post processing was achieved, and the complicated and individualized feeding/draining pattern of AVM can be visualized (Figure 1). It was verified that the hemodynamics of AVM could not be reflected by the widely used surgical Spetzler-Martin grading. Primary flow and further derived parameters were comprehensively evaluated (Figure 2), and the WSS adjacent to the nidus seems to correlate with the venous draining, which required further validation.Conclusion
Cerebral AVM lesions tend to have heterogeneous hemodynamics even with the same Spetzler-Martin grade or similar angioarchitecture, which could be captured and quantified by the individual assessment using 4D flow MR. In this small group of patients, AVM tend to have smaller flow of the feeding artery after rupture. Feeding by single artery was associated with a ruptured lesion. AVM with deep vein drainage tends to have diffusely distributed nidus and higher WSS adjacent to the nidus. Higher flow of the feeding artery was associated with higher DP and larger volume of the nidus.Acknowledgements
/References
1. Fu M, Peng F, Zhang M, et al. Aneurysmal wall enhancement and hemodynamics: pixel-level correlation between spatial distribution. Quant Imaging Med Surg 2022; 12(7):3692-3704.
2. Fennell VS, Martirosyan NL, Atwal GS, et al. Hemodynamics associated with intracerebral arteriovenous malformations: the effects of treatment modalities. Neurosurgery 2018;83:611–21
3. Takeda Y, Kin T, Sekine T, et al. Hemodynamic Analysis of Cerebral AVMs with 3D Phase-Contrast MR Imaging. Am J Neuroradiol. 2021 Dec;42(12):2138-2145.
4. Maria Aristova, Alireza Vali, Sameer A Ansari, et al. Standardized Evaluation of Cerebral Arteriovenous Malformations Using Flow Distribution Network Graphs and Dual-venc 4D Flow MRI. J Magn Reson Imaging. 2019 Dec;50(6):1718-1730