3813
Evaluation of renal allograft function using arterial spin labeling and blood oxygen level-dependent imaging1The First School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, China, 2Department of Interventional Therapy, Chenggong Hospital, Xiamen University, Xiamen, Fujian, China, 3Office of Medical Case Statistics, Department of Medical Affairs, Zhongshan Hospital (Xiamen), Fudan University, Xiamen, Fujian, China, 4Department of Radiology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, Jiangsu, China, 5Department of Radiology, Jinling Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, China, 6GE Healthcare, Beijing, China
Synopsis
Keywords: Kidney, Quantitative Imaging, Arterial spin labelling, Blood oxygen level-dependent imaging, Renal allograft
In this study, we aimed to assess the renal allografts function after transplantation using ASL and BOLD MRI. We found that RBF and R2* values were significantly lower and higher in the renal transplant injury group, respectively. RBF and R2* were positively and negatively correlated with eGFR, respectively. ROC analysis demonstrated both RBF and R2* reflected injured renal function, and the combination of RBF and R2* showed an AUC of 0.86, comparable to that of RBF alone. In conclusion, compared to BOLD, ASL may be a more promising imaging technique for noninvasive assessment of renal graft function for clinical purposes.Background
Kidney transplantation has become a well-established method for the end-stage treatment of patients with chronic renal failure and uremia [1]. Continuous improvement of surgical methods and postoperative monitoring has also greatly improved the 1-year and 5-year survival rates as well as the quality of life of kidney transplant patients with end-stage renal disease [2].Current clinical follow-up methods include SCr measurement, ultrasonography, CT, MRI, and biopsy. Blood oxygen level-dependent (BOLD) MRI can noninvasively assess the distribution of blood oxygen levels in transplanted kidneys, thus reflecting their internal oxygenation status [3-4]. It has been used to reveal the postoperative changes in blood oxygen levels in donor-preserved and recipient kidneys after transplantation, thus confirming its feasibility and applicability in the follow-up renal function monitoring [5]. Additionally, arterial spin labeling (ASL) MRI can be used to obtain perfusion information without the introduction of contrast agents, making it uniquely suited for post-transplant applications. Using ASL, previous studies have found significantly lower perfusion levels in the hypoplastic group by comparing the perfusion of different functional transplanted kidneys [6], and confirmed its reproducibility in post-transplant perfusion detection [7-8].
BOLD and ASL techniques can provide information on morphology, microscopic oxygen metabolism, and renal blood flow, offering a useful tool in detection of graft function during the follow-up. Therefore, here we aimed to assess the function of renal allografts using these two techniques. Furthermore, we investigated the correlation of ASL and BOLD with eGFR, which can be used to explain post-operative pathophysiological changes in the transplanted kidney.
Methods
This retrospective study was approved by the institutional ethics review committee of our hospital. Written informed consent was obtained from all participants. A total of 135 patients (103 males; age: 38.59 ± 11.03 years; age range: 1271 years) were included in this study. SCr concentrations were obtained on the day of MRI and eGFR was calculated. Patients were divided into two groups based on the eGFR values: (1) normal renal allograft function group (eGFR 60 ml/min/1.73 m2; n = 42); (2) renal allograft injury group (eGFR < 60 ml/min/1.73 m2; n = 93). MRI examinations were performed on a 3.0T MRI system (Discovery MR750; GE Healthcare, Milwaukee, WI). ASL imaging was obtained using a 3D pCASL sequence with spiral FSE readout, and with following parameters: TR/TE = 4629/10.5 ms; FOV = 34 × 34 cm2; matrix = 512 × 8 arms; slice thickness/gap = 4/0 mm; labeling duration = 1500 ms; post labeling delay = 1500 ms. BOLD imaging was acquired using a 2D multi-echo GRE sequence with following parameters: TR/TE = 200/2.1–32.8 ms; flip angle = 60 degrees; FOV = 36 × 36 cm2; matrix = 128 × 128; slice thickness/gap = 6/1 mm. RBF and R2* maps were calculated using Advantage Workstation 4.6 (GE Healthcare) from ASL and BOLD images, respectively. For each participant, 6–10 circular ROIs were manually placed in the medulla, and the mean RBF and R2* values were recorded.Statistical analyses were performed using SPSS 25.0 (IBM Corp, Armonk, NY). Chi-square test and independent sample t-test were used to compare differences of categorical and continuous variables between two groups, respectively. Pearson correlation coefficients were calculated to analyze the relationship of RBF and R2* values with eGFR. ROC analysis was performed to assess the diagnostic efficiency of RBF, R2* values, and their combination in distinguishing allografts with injury from those with normal function. Statistical significance was set at p < 0.05.
Results
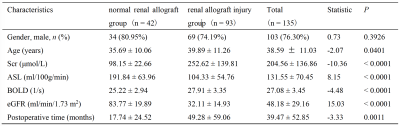
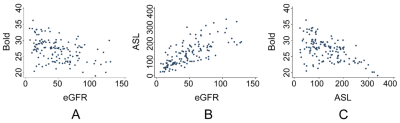
Clinical characteristics of the subjects are shown in Table 1. Patients in the renal allograft injury group were older than those with normally functioning allografts, whilst the renal allograft injury group had longer postoperative time, higher SCr levels, and lower eGFR levels. The RBF value in the renal allograft injury group was significantly decreased, while the R2* value was significantly increased.Figure 1 shows the results of the correlation analysis. RBF values were positively correlated with eGFR (r = 0.73, p < 0.0001), while R2* values were negatively correlated with eGFR (r = -0.44, p < 0.0001). Additionally, a negative correlation was found between RBF and R2* values (r = - 0.54, p < 0.0001).
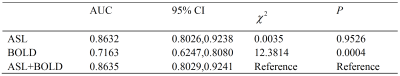
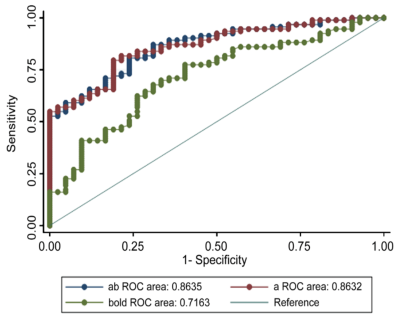
The diagnostic efficiency results in distinguishing renal allografts with injury from normal allografts are shown in Table 2 and Figure 2. The AUC was higher for RBF value (AUC = 0.86) than R2* value (AUC = 0.72). The combination of RBF and R2* showed a significantly higher AUC (AUC = 0.86, p < 0.01) than R2* alone.
Conclusion
Renal transplantation is the treatment of choice for patients with end-stage kidney disease, providing a survival benefit regardless of the graft source. Considering the substantial morbidity associated with graft failure, techniques for detection of renal injury and its timing are key priorities for research. The findings of this study suggested significant differences in RBF and R2* values between patients with normal and injured renal allografts. Both RBF and R2* values can predict renal allograft function injury. RBF value alone exhibited similar diagnostic performance as combined RBF and R2* values. Compared to BOLD, we demonstrated ASL was a more promising imaging technique for noninvasive detection of renal allograft function.Acknowledgements
No acknowledgement found.References
1. Molema, G., and W.C. Aird, 2012. Vascular heterogeneity in the kidney. Semin Nephrol, 32(2): pp. 145-55.
2. Cantow, K., et al., 2021. Quantitative Assessment of Renal Perfusion and Oxygenation by Invasive Probes: Basic Concepts. Methods Mol Biol. 2216: pp. 89-107.
3. Ku, M.C., et al., 2021. Noninvasive Renal Perfusion Measurement Using Arterial Spin Labeling (ASL) MRI: Basic Concept. Methods Mol Biol. 2216: pp. 229-239.
4. Cai, Y.Z., et al., 2017. Diagnostic value of renal perfusion in patients with chronic kidney disease using 3D arterial spin labeling. J Magn Reson Imaging, 46(2): pp. 589-594.
5. Buchanan, C.E., et al., 2020. Quantitative assessment of renal structural and functional changes in chronic kidney disease using multi-parametric magnetic resonance imaging. Nephrol Dial Transplant,35(6): pp. 955-964.
6. OPTN/SRTR 2020. 2018 Annual Data Report: Introduction. Am J Transplant, 20 Suppl s1: pp. 11-19.
7. Langewisch, E. and R.B. Mannon, 2021. Chronic Allograft Injury. Clin J Am Soc Nephrol, 16(11): pp. 1723-1729.
8. Jiang, K., C.M. Ferguson, and L.O. Lerman, 2019. Noninvasive assessment of renal fibrosis by magnetic resonance imaging and ultrasound techniques. Transl Res, 209: pp. 105-120.