3805
Development of an MR Imaging Protocol for Ex-Vivo Assessment of Deceased Donor Human Kidneys1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2Nuffield Department of Surgical Sciences, Oxford University, Oxford, United Kingdom, 3Big Data Institute, Oxford University, Oxford, United Kingdom, 4NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham, United Kingdom, 5Developmental Biology and Cancer Department, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom
Synopsis
Keywords: Kidney, Transplantation
Kidney transplantation is the preferred treatment for end-stage kidney disease. Donor kidney viability is currently assessed using donor age, medical history, and serum creatinine, but these have limited predictive power. As part of a study to determine if MRI can be used as an alternative, more accurate, measurement of donor kidney viability, we outline a multiparametric MRI protocol (high resolution structural scans, relaxometry (T1, T2 and T2*) mapping, susceptibility-weighted-imaging (SWI), diffusion-weighted imaging (DWI), and magnetisation transfer ratio (MTR)) to study human whole kidneys that have been declined as transplants. MRI measures will be integrated with 3D-histology and tissue proteomics datasets.
Introduction
Kidney transplantation is the preferred treatment for end-stage kidney disease. However, patients on the transplant list have prolonged haemodialysis with increased morbidity and mortality rates. Deceased donor kidney viability is currently assessed using donor age, medical history, and serum creatinine, but these have limited predictive power1, resulting in potentially viable donor organs not being transplanted. The development of a rapid, non-invasive, assessment method of deceased donor kidney quality ex-vivo prior to transplant would give clinicians confidence in determining the viability of kidney donor organs.Donor kidneys are declined as transplants mainly due to clinical decision that the organ is of insufficient quality to be transplanted (although it may be viable), thus declined kidneys have a spectrum of qualities. The Whole Organ Research initiative and Quality of Organ Donation (QUOD) biobank2 facilitate allocation of declined human organs to research. Here, we outline an MRI protocol to study discarded whole human kidneys, integrating MRI measures with 3D-histology and tissue proteomics datasets to develop an assessment protocol of deceased donor quality in the ADMIRE (Assessing Donor kidneys and Monitoring Transplant Recipients) study.
Methods
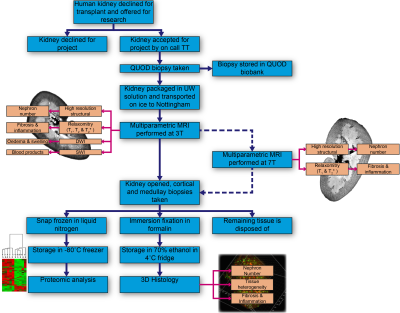
An overview of the research pathway of a discarded kidney is shown in Figure 1.Sample Acquisition
Whole organ research consent is obtained from donor families and declined organs allocated to ADMIRE through the QUOD2 expand program. Donor kidneys are flushed during retrieval to remove any blood, double-bagged in University of Wisconsin preservation solution and further double-bagged in water, then stored in ice in an insulated organ transport box to mimic clinical practice. Organs become available 24-hours a day, and an on-call transplant technician (TT) accepts the organ for research and arranges for delivery to the imaging centre.
MRI Protocol
Prior to scanning, the double bagged kidney is removed from the organ box and placed in an additional watertight bag with fibre optic thermometer probes attached. This allows the temperature of the sample to be monitored during scanning, as it is well known that quantitative MR measures are temperature sensitive.
All kidneys are scanned on a Philips 3T Achieva system in the 32-channel head coil. Prior to studying human kidneys, sequences were optimised using both formalin fixed and fresh porcine kidneys which are of a similar size and structure to human kidneys, to allow effects of temperature and cold ischaemia time to be studied. The MRI protocol consists of multiparametric scans of B0 and B1 field maps, high resolution structural scans, relaxometry (T1, T2 and T2*) mapping, susceptibility-weighted imaging (SWI), diffusion weighted imaging (DWI), and magnetisation transfer ratio (MTR) to assess renal microstructural changes due to fibrosis and inflammation and evaluate blood products remaining within the kidneys. Imaging parameters are outlined in Fig.2. In addition, a subset of kidneys will be scanned on a Philips 7T Achieva to provide higher resolution relaxometry maps, and ultra-high-resolution structural scans for training of super-resolution up-sampling techniques to apply to the 3T datasets.
Histology and Proteomics
A punch biopsy is taken from the kidney following retrieval as part of the national QUOD biobank. After MRI acquisition kidneys are cut lengthwise, and 24 biopsies taken from both cortex and medulla. 12 cortex and medulla samples are formalin fixed for 3D-histology analysis, the remainder are snap frozen for proteomic analysis via the Luminex platform.
Analysis
MRI data are primarily processed using the UKRIN Kidney Analysis Toolbox (UKAT)3,4, with DWI EPI data distortion correction by FSL topup5,6 to allow voxel-wise comparison of the quantitative MRI parameters. A whole kidney mask is generated from the T2-weighted Fast Spin Echo (FSE) image using ITK-Snap7 to estimate total kidney volume. Masks of individual tissue types (cortex, medulla, renal pelvis) are then defined from this scan using a Gaussian mixture model. These masks are applied to the calculation of quantitative parameters for each tissue type.
The location of the QUOD punch biopsy is visible on the structural MRI scan, allowing correlation of histological measures and the Remuzzi donor biopsy score8 with surrounding MRI voxels. Biopsies taken following imaging allow the MRI assessment of the heterogeneity of whole, intact organ kidney tissue microstructure to be linked with kidney pathology.
Results
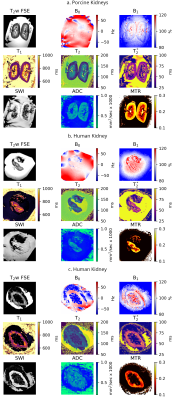
Protocol DevelopmentThe 3T MRI protocol was optimised and tested on fresh porcine kidneys flushed, transported, and stored in an identical way to discarded human kidneys. Resultant high-quality porcine images are shown in Fig.3a. Remaining blood products can clearly be seen in the SWI indicating the flush was not as effective as anticipated.
Human Samples
To date, four human kidneys have been imaged. Example images from one female and one male are shown in Fig.3b and 3c respectively. The volume of the kidney in Fig.3b was 62.1ml; this kidney was small, with the median healthy female left kidney volume in the United Kingdom being 112ml and the 10th percentile being 88ml9. The distribution of quantitative parameters within cortex and medulla are shown in Fig.4.
Conclusion
A MRI protocol has been developed to allow the assessment of donor kidneys, with samples subsequently being collected to allow validation via histology and proteomics. This work aims to establish a framework to allow non-invasive whole kidney assessment to potentially facilitate future transplant graft evaluations.Acknowledgements
This work is funded by Kidney Research UK Grant KS_RP_002_20210111References
1. Watson CJ, Johnson RJ, Birch R, Collett D, Bradley JA. A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation 2012; 93: (3):314-8. doi: 10.1097/TP.0b013e31823f14d4.
2. Quality in Organ Donation (QUOD) https://quod.org.uk
3. Daniel AJ, Nery F, Sousa J, et al. UKRIN Kidney Analysis Toolbox (UKAT): A Framework for Harmonized Quantitative Renal MRI Analysis. In: Proc. Intl. Soc. Mag. Reson. Med. 29. Vol. 29. Online; 2021. p. 3765.
4.Daniel AJ, Nery F, Sousa J, Buchanan C. UKRIN Kidney Analysis Toolbox. 2022 doi: https://doi.org/10.5281/zenodo.4742470.
5. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 2003;20:870–888 doi: 10.1016/S1053-8119(03)00336-7.
6. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23:S208–S219 doi: 10.1016/j.neuroimage.2004.07.051.
7. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006;31:1116–1128 doi: 10.1016/j.neuroimage.2006.01.015.
8. Remuzzi G, Cravedi P, Perna A, Sci S, Dimitrov B, Tarturro M, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med 2006;354(4):343e52. https://doi.org/10. 1056/NEJMoa052891.
9. Langner T, Östling A, Maldonis L, et al. Kidney segmentation in neck-to-knee body MRI of 40,000 UK Biobank participants. Sci. Rep. 2020;10:20963 doi: 10.1038/s41598-020-77981-4.
Figures