3802
The value of 3D APTw Combined Intravoxel Incoherent Motion in Evaluation of the Renal Function in Chronic Kidney Disease1the First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Clinical & Technical Support, Philips Healthcare, China, Shanghai, China
Synopsis
Keywords: Kidney, CEST & MT, amide proton transfer
Early diagnosis of chronic kidney disease (CKD) is difficult because of its hidden condition, mild symptoms and imperceptible symptoms. Amide proton transfer weighted (APTw) is a new contrast-free MR imaging technique, which can non-invasively detect the changes of amide proton concentration in endogenous mobile proteins and polypeptides in tissues. Intravoxel income motion (IVIM) can simultaneously obtain the microcirculation perfusion and water molecule diffusion of patients. The results showed that APTw and IVIM parameters APT, D* and D had good diagnostic efficiency in evaluating CKD renal function, especially the cortex APT value.Introduction
The base of kidney disease patients in the world is huge, with more than 10% of the adult population suffering from chronic kidney disease (CKD). In the early stage of chronic kidney disease, the condition is hidden, the symptoms are mild and imperceptible, and patients often miss the best intervention opportunity, and eventually develop into end-stage kidney disease and even need dialysis treatment or kidney transplantation [1]. Therefore, accurate assessment of kidney structure and function is very important for early diagnosis, early intervention and prognosis evaluation of chronic kidney disease. APTw is a non-invasive and contrast-free imaging based on endogenous proteins and polypeptides in tissues. IVIM is a new MR imaging method that can simultaneously obtain the microcirculation perfusion and water molecule diffusion of patients by using multiple parameters. Both of them have been applied in many diseases of nervous system [2-4]. In this study, APTw and IVIM were used to evaluate the renal function of CKD patients.Methods
A total of 34 patients with CKD who were examined by 3.0 T magnetic resonance scanner (Ingenia CX, Philips Healthcare) in our hospital from August 2019 to April 2022 were collected. The scanning sequences included routine MR examination of kidney, APTw and IVIM (Table.1). According to glomerular filtration rate, they were divided into mild renal damage group (Group A: 18 cases; mean age, 54.44±14.50 years ;age range, 23 -77 years;11 women) and moderate to severe renal damage group (Group B: 16 cases; mean age, 50.19±17.44 years; age range 29 to 78 years; 5 women). Meanwhile, 16 cases of healthy volunteers (Group C: mean age, 50.38±17.88 years; age range, 25-74years; 9 women) were enrolled as health control group. The oval ROIs were placed on the cortex and medulla at the upper, middle and lower pole of the right kidney, and the APT SI, true diffusion coefficient (D), perfusion fraction (f) and pseudo-diffusion coefficient (D*) values were measured (Figure 1). For each parameter, we calculated the average value of the three ROIs of the poles for further analysis. Shapiro-Wilk test was used to analyse the data normality, and Mann-Whitney U test was used to compare the differences between the two groups according to the data normality. Binary Logistic regression and receiver operating characteristic curve (ROC) were used to analyze the diagnostic efficiency of single parameter and combined parameters. Delong test was used to compare the difference among AUC values, and P < 0.05 was considered statistically significant.Results
The analysis of variance (ANOVA) test showed the the medulla D* , cortex D , medulla D , cortex APT, and medulla APT values had significantly difference among the three groups. However, there was no significant difference in cortex D* , cortex f and medulla f values among the three groups (P > 0.05). The APT value of cortex in Group A was significantly higher than that in Group C; The values of medulla D*, cortex D and medulla D in Groups A and C were significantly higher than those in Group B; The APT values of cortex and medulla in Group B were significantly higher than those in groups A and C; The above differences were statistically significant (P < 0.05)(Table 2). Medulla D, D*, APT values and cortex D, APT values, all have good diagnostic efficiency in distinguish group A from group B, group B from group C. However, the combination of APT SI and IVIM-derived parameters showed an decrease of diagnostic performance. Cortex APT has good diagnostic efficiency in distinguishing group A from group C (Table 3). Figure 2 shows the ROC curves for the single and combined parameters in evaluating CKD renal function.Discussion
The APT values reflects the concentration and pH of protein in cells, and some studies have shown that APT values is positively correlated with it [5]. CKD kidney injury is accompanied by the accumulation of extracellular matrix, which is mainly composed of collagen, non-collagen glycoprotein and proteoglycan. Therefore, with the aggravation of kidney injury, protein content increases and APT value gradually increases. As the renal medulla may compensate for some pathological changes when CKD is slightly damaged, there is no significant difference in the APT value of medulla between group A and group C. In CKD, glomerular injury and renal tubular fibrosis lead to decreased renal blood perfusion and water molecule dispersion. Therefore, the cortex D value, medulla D value and medulla D* value in group B are higher than those in groups A and C. The blood flow of cortex is richer than that of medulla, and the compensatory function of kidney is strong, which may be the reason why there is no significant difference between cortex D* value and cortex and medulla f value among the three groups.Conclusion
Our preliminary results showed that APTw and IVIM derived parameters APT, D* and D had good diagnostic efficiency in evaluating CKD renal function. Especially, the cortex APT value, which may be an early predictor of the mild renal damage. These findings indicate that APTw might be a potential noninvasive biomarker for the evaluation of CKD renal function and can provide helpful quantitative MRI information to the disease diagnosis, clinical decision-making and improving prognosis.Acknowledgements
None.References
1. Alnazer I, Bourdon P, Urruty T, et al. Recent advances in medical image processing for the evaluation of chronic kidney disease[J]. Med Image Anal. 2021; 69: 101960. DOI:10.1016/j.media.2021.101960.
2. Zhou J, Heo HY, Knutsson L, van Zijl PCM, Jiang S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J Magn Reson Imaging. 2019 Aug;50(2):347-364. doi: 10.1002/jmri.26645. Epub 2019 Jan 20. PMID: 30663162; PMCID: PMC6625919.
3. Sartoretti E, Sartoretti T, Wyss M, Reischauer C, van Smoorenburg L, Binkert CA, Sartoretti-Schefer S, Mannil M. Amide proton transfer weighted (APTw) imaging based radiomics allows for the differentiation of gliomas from metastases. Sci Rep. 2021 Mar 9;11(1):5506. doi: 10.1038/s41598-021-85168-8. PMID: 33750899; PMCID: PMC7943598.
4. Zou T, Yu H, Jiang C, Wang X, Jiang S, Rui Q, Mei Y, Zhou J, Wen Z. Differentiating the histologic grades of gliomas preoperatively using amide proton transfer-weighted (APTW) and intravoxel incoherent motion MRI. NMR Biomed. 2018 Jan;31(1):10.1002/nbm.3850. doi: 10.1002/nbm.3850. Epub 2017 Nov 3. PMID: 29098732; PMCID: PMC5757627.
5. Ju Y, Liu A, Wang Y, Chen L, Wang N, Bu X, Du C, Jiang H, Wang J, Lin L. Amide proton transfer magnetic resonance imaging to evaluate renal impairment in patients with chronic kidney disease. Magn Reson Imaging. 2022 Apr;87:177-182. doi: 10.1016/j.mri.2021.11.015. Epub 2021 Dec 1. PMID: 34863880.
6. Ma C, Tian S, Song Q, Chen L, Meng X, Wang N, Lin L, Wang J, Liu A, Song Q. Amide Proton Transfer-Weighted Imaging Combined With Intravoxel Incoherent Motion for Evaluating Microsatellite Instability in Endometrial Cancer. J Magn Reson Imaging. 2022 Jun 23. doi: 10.1002/jmri.28287. Epub ahead of print. PMID: 35735273.
Figures
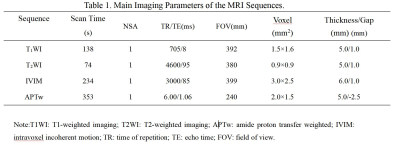
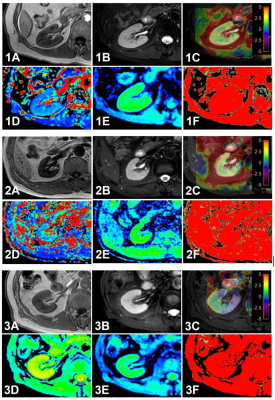
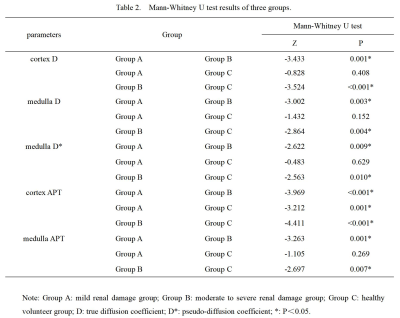
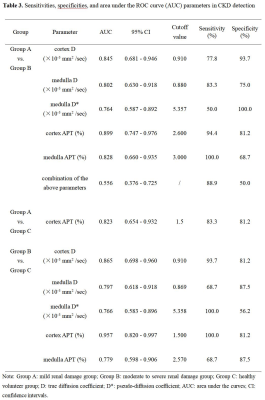
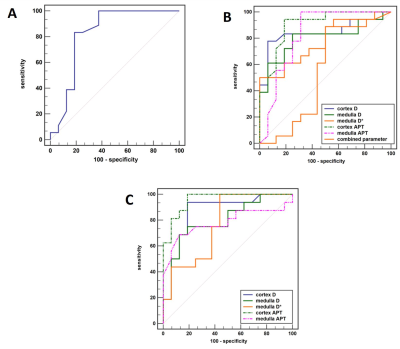
Figure 2.
A. ROC curve of diagnostic efficacy of cortex APT in diagnosing mild renal damage group from healthy volunteers.
B. ROC curve of diagnostic efficacy of cortex D, medulla D, medulla D*, cortex APT , medulla APT and their combination in diagnosing mild renal damage group from moderate to mild renal damage group.
C. ROC curve of diagnostic efficacy of cortex D, medulla D, medulla D*, cortex APT and medulla APT in diagnosing moderate to mild renal damage group from healthy volunteers.