3798
Denoising Enables Faster 1 mm Isotropic Diffusion Tensor Imaging of the Human Hippocampus at 3T
Pablo Stack-Sanchez1, Donald Gross2, and Christian Beaulieu1
1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Neurology, University of Alberta, Edmonton, AB, Canada
1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Neurology, University of Alberta, Edmonton, AB, Canada
Synopsis
Keywords: Data Processing, Diffusion Tensor Imaging
A hippocampus-focused, 1 mm isotropic, diffusion tensor imaging 5.5 minutes 3T protocol has yielded high-quality diffusion images and maps. Post-processing with denoising can potentially reduce the scan time considerably. Compared to the previously used 10 direction/10 average protocol, denoising enabled the use of 4 averages for a scan time of only 2.2 minutes to yield acceptable 1 mm isotropic diffusion image quality and mean diffusivity maps (MD) of the whole hippocampus in healthy controls. This rapid protocol was then able to delineate focal and small hippocampal lesions with elevated MD in temporal lobe epilepsy patients.
Introduction
A practical high-resolution diffusion tensor imaging (DTI) protocol has enabled 1 mm isotropic diffusion images/maps of the human hippocampus in a scan time of 5.5 minutes at 3T1. This protocol has shown hippocampal changes with age in normal development and aging2, and focal elevated mean diffusivity (MD) lesions in temporal lobe epilepsy (TLE)3.Diffusion weighted images (DWI) are limited by their inherent low signal-to-noise ratio (SNR) that can lead to biased estimation of the diffusion parameters4. Denoising algorithms have been used to improve SNR due to their capacity to deal with the non-Gaussian nature of noise. These techniques exploit the structural redundancy across multiple diffusion images to discriminate between relevant information and noise5,6. There are a few human brain diffusion studies showing that denoising can be used to accelerate acquisition times7,8. Some of these methods have been applied to whole brain high spatial resolution data to improve DTI estimation9,10; however, denoising has not yet been applied to the 1 mm hippocampus-focused DTI protocol.
The purpose here was to use denoising in post-processing to reduce the number of averages and hence scan time needed to yield acceptable high-resolution 1 mm isotropic DWI and MD maps in healthy controls, and for the detection of focal lesions in TLE.
Methods
DTI of the hippocampus was acquired in 18 healthy controls (18-73 years, 45±14 years) and 14 TLE patients (18-70 years, 44±13 years) on a 3T Siemens Prisma with a 64-channel head coil using: single-shot spin-echo EPI with Stejskal-Tanner diffusion encoding, 20 1 mm axial-oblique slices aligned along the length of the hippocampus, 1x1 mm2 in-plane with no interpolation, GRAPPA R=2, 6/8 PPF, TE=74 ms, TR=2900 ms, 10 directions and 10 averages, b = 500 s/mm², diffusion time = 29 ms, 5:29 min scan time. Images were denoised with Non Local Spatial and Angular Matching (NLSAM), which was chosen amongst others due to its robustness to both stationary and non-stationary Rician and non-central chi-distributed noise6. Images were then corrected for Gibbs-ringing, eddy current and motion artifacts, and tensor calculation with MRtrix311. The diffusion images were also processed without denoising for comparison.In the 18 controls, the number of averages of the denoised images was systematically reduced as subsets from 10 averages to 1 average, in steps of 1, using the same 10 directions. Image quality was assessed for delineation of internal features of the hippocampus (like the dark band of the Stratum Lacunosum Moleculare, SLM). A whole hippocampus region-of-interest (ROI) was obtained using a convolutional neural network12. for quantitative assessment including mean, standard deviation (STD), and mean square error (MSE, using as reference the 10 averages with denoising) of MD and fractional anisotropy (FA) across all controls (left and right combined). The visualization of focal regions of high MD were compared in the 14 TLE patients between the original 10 averages with denoising and the 4 averages subset with denoising (chosen as shown in Results).
Results
Hippocampal internal structure is clearly depicted in all of the mean DWI (b500) maps with and without denoising, even for as low as 2 averages, although denoising does cause some blurring (Figure 1A). The 10 averages without denoising yields abnormally low MD voxels within the hippocampus (blue voxels, MD <0.6x10-3 mm2/s) and excessively high FA values that get exacerbated with fewer averages; these biased low MD and high FA voxels are reduced with denoising, even with only 4 averages (Figure 1B, C). Mean MD for all controls is almost constant across averages, while mean FA is elevated when reducing the number of averages (Figure 2A, B). Based on the STD and MSE versus averages for the denoised images, 4 averages of 10 diffusion directions appears suitable to accelerate scan time to only 2.2 minutes (Figure 2C-F).The denoised 4 average/10 direction 1 mm isotropic 2.2 min DTI was able to visualize loss of internal contrast (i.e. dark band of SLM) on mean DWI which corresponded to high MD in a TLE patient (Figure 3). Hippocampus MD maps of 3 TLE patients with 10 and 4 averages with denoising are almost identical, supporting a reduction in the number of averages for focal lesion detection (Figure 4).
Discussion
High spatial resolutions are needed to visualize small, complex brain structures such as the hippocampus, but this results in long scan times (averaging) and the low SNR can cause bias in diffusion tensor parameters. Denoising was shown here to enable a marked reduction in scan time (60%) for a previously published 1 mm isotropic hippocampus-coverage DTI protocol1 from 5.5 minutes to only 2.2 minutes, which makes it more clinically applicable. The denoised images are slightly less sharp, but the hippocampal internal structure is still clearly depicted, have fewer biased low MD and high FA values, and focal lesions are readily visualized in epilepsy patients. One specific denoising algorithm (NLSAM6) was chosen here, but others could be compared as well for this particular application5-10. In conclusion, it is possible to acquire 1 mm isotropic DTI of human hippocampus in 2.2 minutes at 3T and use it to assess lesions in TLE patients.Acknowledgements
No acknowledgement found.References
- Treit S, Steve T, Gross DW, Beaulieu C. High resolution in-vivo diffusion imaging of the human hippocampus. Neuroimage. 2018;182:479–87.
- Solar KG, Treit S, Beaulieu C. High resolution diffusion tensor imaging of the hippocampus across the healthy lifespan. Hippocampus. 2021;31(12):1271–84.
- Treit S, Little G, Steve T, Nowacki T, Schmitt L, Wheatley BM, et al. Regional hippocampal diffusion abnormalities associated with subfield-specific pathology in temporal lobe epilepsy. Epilepsia Open. 2019;4(4):544–54.
- Pierpaoli C, Basser PJ. (1996), Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med., 36: 893-906.
- Manjón J V., Coupé P, Concha L, Buades A, Collins DL, Robles M. Diffusion Weighted Image Denoising Using Overcomplete Local PCA. PLoS One. 2013;8(9):1–12.
- St-Jean S, Coupé P, Descoteaux M. Non Local Spatial and Angular Matching: Enabling higher spatial resolution diffusion MRI datasets through adaptive denoising. Med Image Anal. 2016;32(Umr 5800):115–30.
- Gramfort A, Poupon C, Descoteaux M. Denoising and fast diffusion imaging with physically constrained sparse dictionary learning. Med Image Anal. 2014;18(1):36–49.
- Kawamura M, Tamada D, Funayama S, Kromrey ML, Ichikawa S, Onishi H, Motosugi U. Accelerated acquisition of high-resolution diffusion-weighted imaging of the brain with a multi-shot echo-planar sequence: deep-learning-based denoising. Magn Reson Med Sci. 2021;20(1):99–105.
- Moeller S, Pisharady PG, Ramanna S, Lenglet C, Wu X, Dowdle L, Yacoub E, Uğurbil K, Akçakaya M. Noise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing,. NeuroImage. 2021;226: 117539.
- Ma X, Uğurbil K, Wu X. Denoise magnitude diffusion magnetic resonance images via variance-stabilizing transformation and optimal singular-value manipulation. NeuroImage. 2020;215:116852.
- Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh C-H, Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137.
- Efird C, Neumann S, Solar KG, Beaulieu C, Cobzas D. Hippocampus segmentation on high resolution diffusion MRI. Proc - Int Symp Biomed Imaging. 2021;2021:1369–72.
Figures
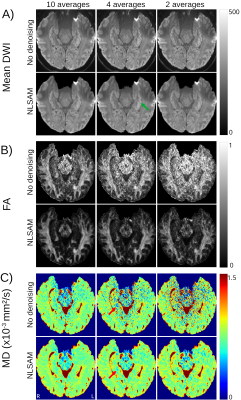
Mean DWI (b500), FA, and MD maps in a control. Denoised (NLSAM) mean DWI are slightly less sharp, nevertheless, the internal structure of the hippocampus is clearly depicted (green arrow) even with fewer averages. The biased (B) high FA (white voxels) and (C) low MD (blue voxels) voxels within the hippocampus are reduced with denoising (red arrow). Both MD and FA maps are almost identical between 10 and 4 averages with denoising.
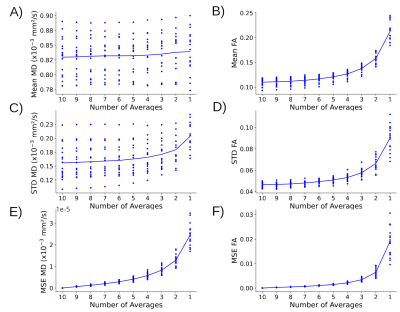
Mean, standard deviation (STD), and mean square error (MSE) for MD (column 1) and FA (column 2) across the whole hippocampus (left and right) in 18 controls with 10 to 1 averages for NLSAM denoised images. (A) Mean MD is stable across all averages whereas (B) mean FA gets larger with fewer averages. (C-F) STD and MSE increase greatly after 3 averages for both MD and FA. Hence, the number of averages to accelerate scan time was chosen to be 4 here.
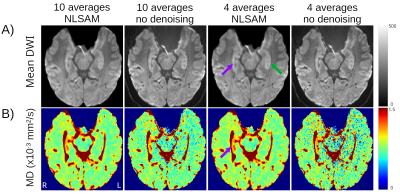
Mean DWI (b500) and MD maps with 10 or 4 averages with and without denoising in a TLE patient with right hippocampal sclerosis. The loss of internal structure (notably the dark line indicating the SLM on the contralateral side (green arrow)) of the ipsilateral hippocampus on mean DWI (purple arrow) is visible in all cases. This region corresponds to elevated MD which is readily observed with 4 averages and denoising.
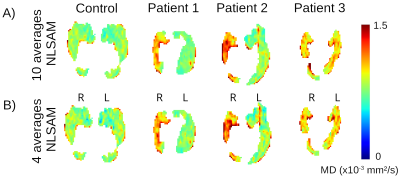
1 mm isotropic hippocampus MD maps of 1 control and 3 example TLE patients acquired with 10 and 4 averages of 10 directions with denoising. Both protocols detect similar regions of greater MD that indicate focal lesions that differ in spatial location and extent in the TLE patients.
DOI: https://doi.org/10.58530/2023/3798