3789
Spatiotemporal signal drift in diffusion MRI of the brain and ways to correct for it: effects on estimates of ADC and IVIM f1Department of Medical Sciences, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
Synopsis
Keywords: Data Processing, Diffusion/other diffusion imaging techniques
Signal drift has been identified as a confounding factor in diffusion MRI (dMRI), causing increases variance and potential bias in derived parameter maps. In this work, we show that temporal signal drift is spatially dependent in human brain images for dMRI, thus calling for spatiotemporal corrections. We also show that signal drift can have a substantial effect on short-term repeatability of ADC and IVIM f obtained from data acquired with a protocol ordered by b-value as is commonly done in clinical practice.Introduction
Signal drift has been identified as a confounding factor in diffusion MRI (dMRI), causing increases variance and potential bias in derived parameter maps1,2. Global signal drift was observed and corrected for in phantom and human brain by Vos et al.1, while spatially varying signal drift in phantom later was addressed by Hansen et al.2. In this work, we study the spatial variations in signal drift for dMRI of the human brain and ways to correct for it, as well as the effects on estimates of the apparent diffusion coefficient (ADC) and the intravoxel incoherent motion (IVIM) perfusion fraction (f)3.Methods
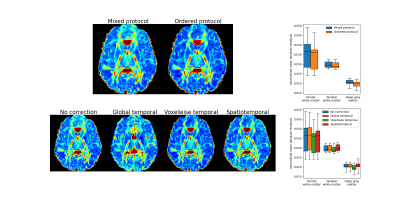
Diffusion-weighted images of the brain in six healthy volunteers (6 males, 21-25 years) were acquired on a Philips MR7700 3T MR scanner with a 32-channel head coil. The study was approved by the Swedish ethical review authority (Dnr 2020-00029). The imaging protocol included three b-values (0, 200, 800 s/mm2) and six diffusion encoding direction (sides of a cube), with repetitions of b-values according to the ratio 2:3:1 given by optimization of an IVIM acquisition in the brain4. Repeatability was assessed by a second equal scan in direct succession.Signal drift was assessed and corrected for using the b=0 images, which are intended to be equal throughout a scan. Three correction methods were applied 1) a global temporal polynomial correction where a 2nd order polynomial was fitted to the brain median signal value of each b=0 image and then applied to the full scan as described by Vos et al.1, 2) a voxelwise temporal polynomial correction similar to the first method, but where a separate polynomial was fitted for each voxel, and 3) a spatiotemporal polynomial correction where instead a 2nd order polynomial in both time and space was used as described by Hansen et al.2.
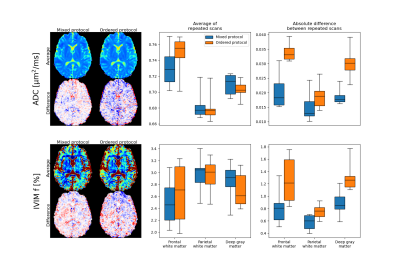
The influence of acquisition order was evaluated by comparison of data acquired from the original protocol with b-values and diffusion encoding directions mixed (mixed protocol), to data corresponding to a protocol ordered by b-value and with all diffusion encoding directions acquired in direct succession for each b-value separately (ordered protocol). Data for the ordered protocol was generated by applying the inverse voxelwise temporal polynomial correction to corrected data after a reordering according to b-value.
Parameter maps of the apparent diffusion coefficient (ADC) were estimated from images with b-values 200 and 800 s/mm2, and the extrapolated signal at b=0 in combination with the measured signal at b=0 was used to estimate the IVIM perfusion fraction (f)5.
Results
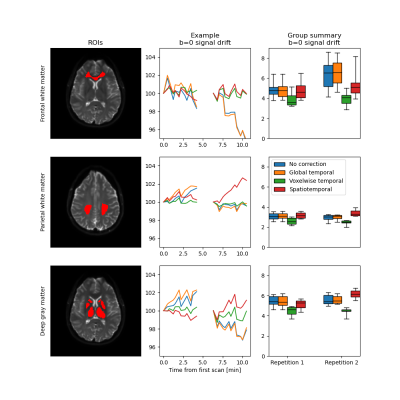
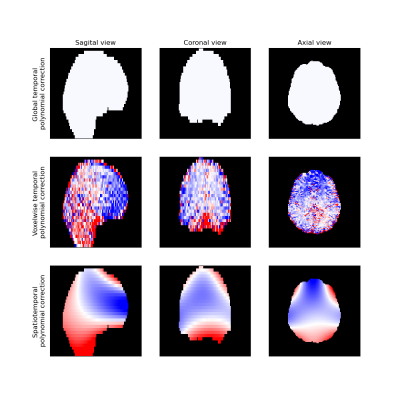
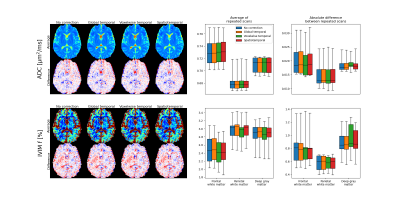
Signal drift with a magnitude of around five percent over a single scan was observed in all subjects (Fig. 1). There were distinct spatial variations in signal drift which could not be corrected for with the global temporal polynomial correction, while the voxelwise temporal polynomial correction appeared to produce stable results (Figs. 1 and 2). The spatiotemporal polynomial correction was, in general, able to capture the overall spatial variations in signal drift as seen when compared with the voxelwise temporal polynomial correction (Fig. 2), but appeared less stable and therefore failed to produce the corresponding reduction in signal drift after correction (Fig. 1).The acquisition order had substantial effects on how signal drift affected the repeatability of estimated parameters. Both ADC and IVIM f had about 50 % larger variations between repeated scans for the protocol where acquisitions were ordered by b-value as compared with the protocol where b-values and diffusion encoding directions were mixed (Fig. 3). The choice of correction method had little effect on repeatability for the mixed protocol (Fig. 4). Weak trends towards smaller residuals could be seen for the order protocol compared with the mixed protocol, and for the voxelwise temporal polynomial correction compared with the other correction methods (Fig. 5).
Discussion
The magnitude of the signal drift observed in the current study was similar to that reported by Vos et al. even though their scans were three times as long1. This might be explained by the observation that signal drift can be of opposite signs in different parts of the brain. Analysis of global averages may thus result in underestimation of the local signal drifts.The spatiotemporal polynomial correction suggested by Hansen et al.2 appeared to be able to capture the overall pattern as seen in Figure 2, but was also associated with overcompensations as seen in Figure 1. The implementation suggested by Hansen et al. is intended to be robust to outliers, but additional improvements might be needed to avoid overfitting to particular voxels, e.g. those in the rim of the brain.
The weak trend towards smaller residuals for the ordered protocol is expected as signal drift has a smaller impact per b-value for an ordered protocol, for which drift instead introduces a bias. Similarly, smaller residuals for the voxelwise temporal polynomial correction can be explained by reduced signal variations per b-value.
Conclusion
Temporal signal drift in images for dMRI is spatially dependent, which calls for spatiotemporal corrections. Signal drift can have a substantial effect on short-term repeatability of ADC and IVIM f obtained from data acquired with a protocol ordered by b-value as is commonly done in clinical practice.Acknowledgements
The study was financed by grants from the Assar Gabrielsson Foundation, the Sahlgrenska University Hospital Research Fund, the Royal Society of Arts and Sciences in Gothenburg (KVVS), and the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement.References
1. Vos SB, Tax CMW, Luijten PR, et al. The importance of correcting for signal drift in diffusion MRI. Magn. Reson. Med. 2017;77(1):285–299.
2. Hansen CB, Nath V, Hainline AE, et al. Characterization and correlation of signal drift in diffusion weighted MRI. Magn. Reson. Imaging 2019;57(July 2018):133–142.
3. Le Bihan D, Breton E, Lallemand D, et al. Separation of Diffusion and Perfusion in Intravoxel Incoherent Motion MR Imaging. Radiology 1988;168(2):497–505.
4. Jalnefjord O, Montelius M, Starck G, et al. Optimization of b-value schemes for estimation of the diffusion coefficient and the perfusion fraction with segmented intravoxel incoherent motion model fitting. Magn. Reson. Med. 2019;82(4):1541–1552.
5. Jalnefjord O, Andersson M, Montelius M, et al. Comparison of methods for estimation of the intravoxel incoherent motion (IVIM) diffusion coefficient (D) and perfusion fraction (f). Magn. Reson. Mater. Physics, Biol. Med. 2018;31:715–723.
Figures