3782
Fast mean kurtosis measurements using navigator-free 3D multi-slab DWI1Department of Radiology, The University of Iowa, Iowa City, IA, United States
Synopsis
Keywords: Data Acquisition, Diffusion/other diffusion imaging techniques, Kurtosis; Multi-slab; 3D
The fast mean kurtosis measurements using 2D DWI has reduced the scan time substantially but remains subject to low SNR, particularly at b-value = 2500 s/mm2. A larger voxel (≥ 2 mm3) is normally used to increase SNR. This study presents the navigator-free 3D multi-slab DWI for fast mean kurtosis measurements with a resolution of 1.5 mm3. By using a short TR of 2.5 sec, the work demonstrates the promise of using the navigator-free 3D multi-slab DWI for whole-brain fast mean kurtosis measurements with an improved SNR efficiency.
INTRODUCTION
Diffusion kurtosis imaging (DKI)1 has been shown to provide more information on the brain microstructure than the diffusion tensor imaging (DTI) and is potentially useful in many clinical applications2. A recently proposed method3 allows a fast mean kurtosis measurement by using only 13 images (b0 + 12 DWI images), resulting in substantially reduced scan time and facilitating the translation of DKI into clinics. However, DKI remains subject to low SNR, particularly at the b-value = 2500 s/mm2. A larger voxel size (≥ 2 mm3) is typically used for a whole-brain DKI to increase SNR but at the cost of image resolution3-8. One approach to increase the SNR efficiency is to shorten the TR through the 3D multi-slab DWI, where a whole-brain, high-resolution DWI (< 2 mm3) can be attained with a short TR around 2 sec9-17. Previous studies have demonstrated the application of the 3D multi-slab DWI for DTI9-17. The utilization of the 3D multi-slab DWI for mean kurtosis measurements has not been investigated. Therefore, the purpose of this study is to investigate the feasibility of using the navigator-free 3D multi-slab DWI17 for whole-brain fast mean kurtosis measurements with a resolution of 1.5 mm3.METHODS
Sequence:The 3D multi-slab DWI was developed by integrating a phase-encoding blip along the slice direction into a 2D echo planar imaging (EPI) DWI sequence as described in previous works9-17. Both the excitation and refocusing pulses were designed using the Shinnar-Le Roux algorithm18 with a time-bandwidth product of 12 to attain sharp RF profiles10.
Reconstruction:
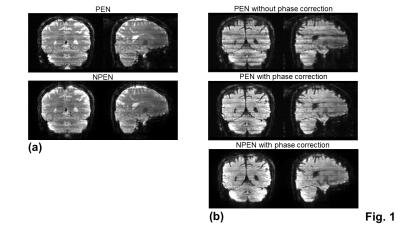
After obtaining the 3D k-space data, a 2-D SENSE algorithm19,20 was used to obtain a full-sampled, coil combined data for each kz plane of each slab. For b0 images, a 3-D Fourier transform was applied to each slab to produce 3D slab images. For DWI images, the inter-shot phase variations were present between kz-planes within a slab due to motion. Therefore, a navigator-free method, known as the self-navigation correction method16, was applied to each kz plane to correct the motion-induced phase errors prior to a 3-D Fourier transform. The 3D slab images were then combined into a single 3D volume using PEN13 and NPEN14 to mitigate the slab boundary artifacts.
In vivo experiment:
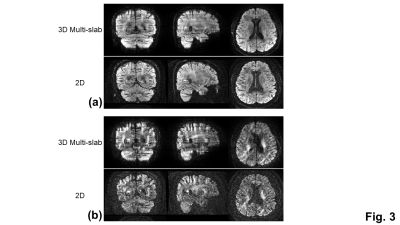
All MRI scans were performed on a 3 Tesla SIGNA Premier system (GE Healthcare, Milwaukee, Wisconsin) equipped with a 48-channel head receive coil and a 80 mT/m gradient system. The imaging protocol, including one DTI measurement (maximal b-value:1000 s/mm2, b0 + 6 directions) and one mean kurtosis measurements (maximal b-value: 2500 s/mm2, b0 + 12 directions), was performed on a healthy volunteer. Imaging parameters were: in-plane FOV of 216 mm, in-plane matrix of 144 x 144, in-plane acceleration of 2 along ky, pixel bandwidth of 1953 Hz, TR of 2500 ms, slab thickness/overlap of 15/1.5 mm, slab FOV of 21 mm, 14 z-encodes (40% oversampling), 11 slabs, resolution of 1.5 mm3, partial Fourier encoding factor of 0.72, and TE of 70 ms for DTI measurements and 82 ms for mean kurtosis measurements. The scan times for DTI and mean kurtosis measurements were 4 min 8 sec and 7 min 38 sec, respectively. For comparison, the 2D EPI DWI was also used for mean kurtosis measurements with the same brain coverage (100 slices), resolution, TE and scan time as the 3D multi-slab DWI. The TR of the 2D EPI DWI was 17000 ms. The number of averages for the 2D EPI DWI was 2.
Analysis:
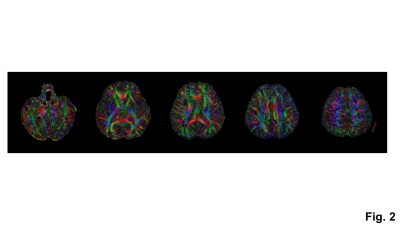
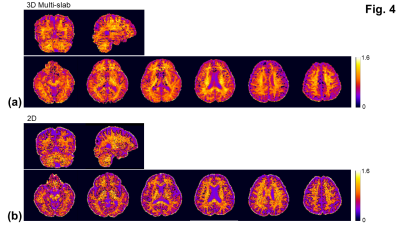
DTI processing and the computation of color-coded FA maps were performed using DSI studio21. The mean kurtosis maps were computed using the method described previously3,4,22.
RESULTS and DISCUSSION
With the phase correction, the signal drop in DWI images was mitigated (Fig. 1b). The remaining slab boundary artifacts were reduced by using NPEN for combining slab images (Figs. 1a and 1b). Following the phase correction and NPEN, the 3D multi-slab DWI images for DTI measurements were used to generate color-coded FA maps (Fig. 2). For DKI measurements, the 3D multi-slab DWI images showed a higher SNR than the 2D DWI images (Fig. 3). The benefit of SNR was demonstrated by the mean kurtosis maps (Fig. 4). The mean kurtosis maps obtained by the 3D multi-slab DWI were more smooth in white-matter regions than those obtained by the 2D DWI. However, many zeros (negative kurtosis) were present on the mean kurtosis maps mainly at the gray matter–white matter and gray matter–CSF interfaces, likely caused by the partial volume effects. In the 3D multi-slab DWI, negative kurtosis may also result from the procedure for correcting the motion-induced phase errors. The self-navigation correction method17 relies on the smooth phase differences between b0 and DWI images to estimate the motion-induced phase. The choice of the smoothing kernel may result in incomplete removal of the phase errors or undesirable removal of the phases related to structural variations9,20,23. Future work is to increase the image resolution to reduce the partial volume effects and structure-related phases, and allow a more complete removal of the phase errors.CONCLUSION
This study demonstrates the promise of using the navigator-free 3D multi-slab DWI for whole-brain fast mean kurtosis measurements with an improved SNR efficiency.Acknowledgements
The authors express special thanks to Dr. Wenchuan Wu for sharing the codes of NPEN for combining slab images. This work was supported by the National Institutes of Health R01EB031169. Core facilities were supported in part by the National Institutes of Health S10OD025025 and S10RR028821.References
[1]. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005 Jun;53(6):1432-40.
[2]. Steven AJ, Zhuo J, Melhem ER. Diffusion kurtosis imaging: an emerging technique for evaluating the microstructural environment of the brain. AJR Am J Roentgenol. 2014 Jan;202(1):W26-33.
[3]. Hansen B, Lund TE, Sangill R, Jespersen SN. Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med. 2013 Jun;69(6):1754-60.
[4]. Jensen JH, Hu C, Helpern JA. Rapid data acquisition and postprocessing for diffusional kurtosis imaging. In Proceedings of the 17th Annual Meeting of ISMRM, Honolulu, Hawaii, USA, 2009. Abstract 1403.
[5]. Tietze A, Hansen MB, Østergaard L, Jespersen SN, Sangill R, Lund TE, Geneser M, Hjelm M, Hansen B. Mean Diffusional Kurtosis in Patients with Glioma: Initial Results with a Fast Imaging Method in a Clinical Setting. AJNR Am J Neuroradiol. 2015 Aug;36(8):1472-8.
[6]. Hansen B, Lund TE, Sangill R, Stubbe E, Finsterbusch J, Jespersen SN. Experimental considerations for fast kurtosis imaging. Magn Reson Med. 2016 Nov;76(5):1455-1468.
[7]. Hansen B, Shemesh N, Jespersen SN. Fast imaging of mean, axial and radial diffusion kurtosis. Neuroimage. 2016 Nov 15;142:381-393.
[8]. Henriques RN, Jespersen SN, Jones DK, Veraart J. Toward more robust and reproducible diffusion kurtosis imaging. Magn Reson Med. 2021 Sep;86(3):1600-1613.
[9]. Engström M, Skare S. Diffusion-weighted 3D multislab echo planar imaging for high signal-to-noise ratio efficiency and isotropic image resolution. Magn Reson Med. 2013 Dec;70(6):1507-14.
[10]. Engström M, Mårtensson M, Avventi E, Skare S. On the signal-to-noise ratio efficiency and slab-banding artifacts in three-dimensional multislab diffusion-weighted echo-planar imaging. Magn Reson Med. 2015 Feb;73(2):718-25.
[11]. Bruce IP, Chang HC, Petty C, Chen NK, Song AW. 3D-MB-MUSE: A robust 3D multi-slab, multi-band and multi-shot reconstruction approach for ultrahigh resolution diffusion MRI. Neuroimage. 2017 Oct 1;159:46-56.
[12]. Chang HC, Sundman M, Petit L, Guhaniyogi S, Chu ML, Petty C, Song AW, Chen NK. Human brain diffusion tensor imaging at submillimeter isotropic resolution on a 3Tesla clinical MRI scanner. Neuroimage. 2015 Sep;118:667-75.
[13]. Van AT, Aksoy M, Holdsworth SJ, Kopeinigg D, Vos SB, Bammer R. Slab profile encoding (PEN) for minimizing slab boundary artifact in three-dimensional diffusion-weighted multislab acquisition. Magn Reson Med. 2015 Feb;73(2):605-13.
[14]. Wu W, Koopmans PJ, Frost R, Miller KL. Reducing slab boundary artifacts in three-dimensional multislab diffusion MRI using nonlinear inversion for slab profile encoding (NPEN). Magn Reson Med. 2016 Oct;76(4):1183-95.
[15]. Wu W, Poser BA, Douaud G, Frost R, In MH, Speck O, Koopmans PJ, Miller KL. High-resolution diffusion MRI at 7T using a three-dimensional multi-slab acquisition. Neuroimage. 2016 Dec;143:1-14.
[16]. Dai E, Liu S, Guo H. High-resolution whole-brain diffusion MRI at 3T using simultaneous multi-slab (SMSlab) acquisition. Neuroimage. 2021 Aug 15;237:118099.
[17]. Moeller S, Ramanna S, Lenglet C, Pisharady PK, Auerbach EJ, Delabarre L, Wu X, Akcakaya M, Ugurbil K. Self-navigation for 3D multishot EPI with data-reference. Magn Reson Med. 2020 Oct;84(4):1747-1762.
[18]. Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm [NMR imaging]. IEEE Trans Med Imaging. 1991;10(1):53-65.
[19]. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001 Oct;46(4):638-51.
[20]. Liu C, Moseley ME, Bammer R. Simultaneous phase correction and SENSE reconstruction for navigated multi-shot DWI with non-cartesian k-space sampling. Magn Reson Med. 2005 Dec;54(6):1412-22.
[21]. Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010 Sep;29(9):1626-35.
[22]. Sun PZ, Wang Y, Mandeville E, Chan ST, Lo EH, Ji X. Validation of fast diffusion kurtosis MRI for imaging acute ischemia in a rodent model of stroke. NMR Biomed. 2014 Nov;27(11):1413-8.
[23]. Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med. 2002 Jan;47(1):42-52.
Figures