3768
Extraction of the Utero-Placental and Fetal Vasculature using 2D Time-of-Flight Imaging1Department of Radiology, Wayne State University, Detroit, MI, United States, 2Perinatology Research Branch, NICHD/NIH/DHHS, Detroit, MI, United States, 3Department of Obstetrics and Gynecology, Wayne State University, Detroit, MI, United States
Synopsis
Keywords: Segmentation, Fetus
Mapping the vasculature, flow and tissue properties of the placenta and umbilical cord can serve as a means to study placental and fetal health. The goal of this work is to use a rapid, multi-echo, interleaved GRE sequence to minimize motion artifacts and cover the entire abdomen of the mother in a few minutes. To better map out the vasculature, we propose to both separate the arteries and veins in the umbilical cord using an R2* mapping thresholding technique and use vessel tracking to create 3D renderings. We have successfully done this in a series of 10 fetuses.Introduction
The placenta plays a vital role in providing nutrients and oxygen to the fetus. The exchange process within the cotyledon is driven by the in-flow and out-flow of blood from both the maternal and fetal sides. Prohibition of this exchange process leads to placental dysfunction which is considered to be the leading factor for conditions like fetal growth restriction (FGR) and preeclampsia1-4. It has also been shown that reduced flow in the umbilical cord is a risk factor for premature birth. Hence, the use of MR angiographic (MRA) imaging in pregnancy can not only help in evaluating the normal developing vasculature of the human fetus and placenta but may also help in identifying abnormal development5. In this study, we propose a technique for overcoming motion artifacts using an interleaved gradient recalled echo (GRE) sequence that allows us to acquire time-of-flight (TOF) like images, T2*, T1 and spin density maps. We also propose to separate the arteries and veins in the umbilical cord and visualize the chorionic, fetal and umbilical vessels in 3D using the oxygen saturation effects in the vessels as visualized with T2* maps.Methods
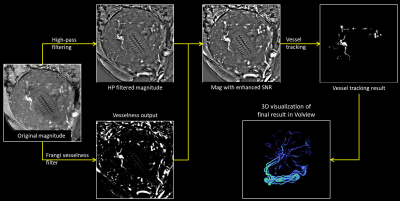
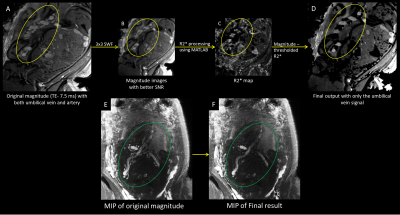
We used a 2D interleaved, multi-echo, GRE imaging sequence with both a low flip angle (proton density weighted) and a high flip angle (T1 weighted with a TOF like effect) within the same sequence in roughly 5 seconds per slice. This makes it possible to quantify tissue characteristics and help separate arteries from veins. Five pregnant women suspected of fetal growth restriction were scanned during their second and third trimester (24 – 37 weeks of gestation) using a 3.0T Siemens Verio scanner. The following imaging parameters were used6: TR = 35-40 ms, TE = 7.5/27.5 ms, FAs = 8°/40°, voxel size = 1×1 mm2 and slice thickness = 3 mm. In order to extract the vascular information, a Frangi vesselness filter7 was applied to the data. The vesselness results were then manually cropped using SPIN (Signal processing in NMR, SpinTech, Detroit, MI) software and added to the high pass filtered magnitude image to obtain an image with higher SNR and improved vascular information. The vessels were then tracked using an automated vessel extraction tool in SPIN software which uses an adaptive thresholding algorithm. The tracked result is then visualized in 3D space using Volview 2.0 software. The vessel extraction process is depicted in Figure 1.Arteries and veins were distinguished using a thresholding method on the R2* maps computed from the two magnitude images (TE 7.5 and 27.5 ms) for each FA (8° and 40°). These two R2* maps were then averaged. A threshold of 30/s-40/s was applied to suppress the umbilical artery signal (deoxygenated blood). The thresholded image was then subtracted from the original image to produce a venous map of the umbilical cord carrying oxygenated blood. The artery-vein separation process is shown in Figure 2.
Results
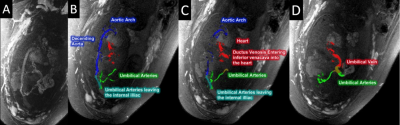
The interleaved sequence provided images showing the usual TOF effect from the high FA interleaved images that were then compared with the conventional TOF sequences acquired with the same resolution. The vessel tracking approach was applied to different regions of the fetal and placental vasculature. Due to the simultaneous acquisition of the multi-echo interleaved GRE data using two FAs, we were able to create high quality R2* maps, to aid in differentiating veins from arteries. When the MRA was acquired covering the entire abdomen of the mother, we were able to visualize the following vascular territories: blood supply of the mother’s uterine arteries; endometrial vessels feeding the placenta; the umbilical cord penetrating the chorionic plate; and the fetal vessels. Figure 3 shows the extraction of fetal vessels including the heart, inferior vena cava, aortic arch, subclavian artery, carotid arteries, jugular veins, and the ductus venosus.Discussion
Using this sequence, we can map the vessels (maternal, fetal and placental), quantify tissue properties using STAGE in just 5 to 10 minutes also alleviating most motion artifacts. Future applications include fully automated vessel extraction in other anatomical regions, monitoring oxygen saturation and study placental health using quantitative susceptibility maps (QSM) maps and T2* data in fetal growth restricted subjects using just one powerful imaging sequence.Conclusion
In this study, we presented a non-invasive approach for acquiring and processing the interleaved multi-echo GRE data that helps in the visualization and quantification of the placental-fetal vascular territory.Acknowledgements
No acknowledgement found.References
1. Krishna U, Bhalerao S. Placental Insufficiency and Fetal Growth Restriction. The Journal of Obstetrics and Gynecology of India. 2011;61(5):505-11.
2. MYATT L, WEBSTER RP. Vascular biology of preeclampsia. Journal of Thrombosis and Haemostasis. 2009;7(3):375-84.
3. Regnault TRH, Galan HL, Parker TA, Anthony RV. Placental Development in Normal and Compromised Pregnancies— A Review. Placenta. 2002;23:S119-S29.
4. Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and Angiogenic Imbalance. Annual Review of Medicine. 2008;59(1):61-78.
5. Neelavalli J, Krishnamurthy U, Jella PK, Mody SS, Yadav BK, Hendershot K, et al. Magnetic resonance angiography of fetal vasculature at 3.0 T. European Radiology. 2016;26(12):4570-6.
6. Chen Y, Liu S, Buch S, Hu J, Kang Y, Haacke EM. An interleaved sequence for simultaneous magnetic resonance angiography (MRA), susceptibility weighted imaging (SWI) and quantitative susceptibility mapping (QSM). Magnetic Resonance Imaging. 2018;47:1-6.
7. Frangi AF, Niessen WJ, Vincken KL, Viergever MA, editors. Multiscale vessel enhancement filtering. Medical Image Computing and Computer-Assisted Intervention — MICCAI’98; 1998 1998//; Berlin, Heidelberg: Springer Berlin Heidelberg.
Figures