3754
Three-dimensional CUBE MR imaging accurately quantified lipid accumulation in reflection of longitudinal fish health1Department of Radiology, Tongji Hosptial of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China, 2Faculty of Resources and Environmental Science, Hubei University, Wuhan, China, 3MR Research, GE Healthcare, Wuhan, China
Synopsis
Keywords: Liver, Animals, assess fish health
Quantitative methods of fish lipid accumulation have attracted a lot of attention in ecotoxicology. Traditionally, invasive approaches were time-consuming and generally required animal sacrifice, drastically restricting applicability in ecotoxicology. In this study, a noninvasive MRI approach was proposed to quantify lipid accumulation in two represented fish species with IDEAL-IQ and Cube-Flex-T2 sequences. We demonstrated the accuracy of our novel MRI approach in quantifying fish lipid accumulation. In addition, we conducted cadmium exposure experiment and observed no significant differences in lipid deposition between MRI and traditional methods. Thus, this MRI method could have a wide range of applications in ecotoxicological research.Background and Purpose
In organisms, lipids are involved in a variety of vital biological processes, such as energy storage, hormone regulation and signal transduction[1]. Appropriate lipid accumulation in fish is crucial for maintaining ecosystem stability, biological diversity and fish health[2]. Lipid accumulation apparently has been thought as a diagnostic biomarker to evaluate the toxic effects of organic and inorganic contaminants in fish in the ecotoxicology[3]. Therefore, accurately and precisely quantifying fish lipid accumulation in fish liver has attracted much attention in ecotoxicology.Recently, novel IDEAL-IQ (Iterative Decomposition of water and fat with the Echo Asymmetry and Least-squares estimation – Intelligence Quotient) and 3D FSE-Cube Flex T2 sequences on in vivo fat quantification and tissue volume measurement, respectively, have recently been applied widely in humans S, but rarely in fish[4].
In the study, we selected two iconic high-economic-value Chinese fish species, the snakehead (Channa argus) and the big-eyed mandarin fish (Siniperca knerii). We tested the reliability and performance of this new MRI approach by comparing MRI results with traditional methods (somatic indices and chemical measurements of lipid content) . In addition, the applicability of this MRI method in quantify lipid accumulation was validated with C. argus experimentally exposed to cadmium (Cd), a commonly identified pollution factor in aquatic environment, in C. argus to test the applicability of this MRI method in ecotoxicological research.
Material and methods
A total of 93 fresh fish, including 43 C. argus and 50 S. knerii were randomly selected and purchased from Guangbutun markets of aquatic products in Wuhan during March-July 2018 and May-September 2019. Fish were periodically brought to the Department of Radiology for MRI measurements.All fish survived after the MRI scans on a 3.0 T clinical MRI scanner (Signa Pioneer; GE Healthcare) with a 48-channel head coil with no negative effects on health. 3D-FSE-Cube-Flex T2 sequence was used to measure fish whole-body volume. The percentage volume of liver (%) was determined from the measured volume of liver and that of the entire body of the fish; fat fraction maps were automatically computed by IDEAL IQ. After MRI examinations, each fish was weighed and dissected to obtain the hepatosomatic index (HSI) as liver weight over whole-fish weight. For chemical process, fish liver samples were analyzed using an standard chemical method. All statistical analyses were performed using R software. Pearson’s correlation analysis was used to assess the relationships between MRI measurements and HSI, IPF, chemical approaches, respectively.Results
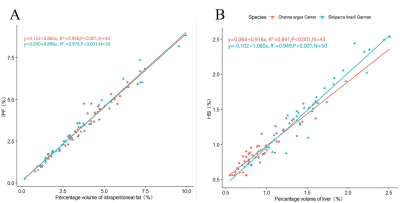
The correlation between percentage volume of intraperitoneal fat measured by MRI and traditional mean IPF was excellent for both C. argus (r= 0.979, P <0.001) and S. knerii (r = 0.989, P <0.001). The regression model showed a high coefficient between the volume of intraperitoneal fat and the traditional mean IPF with adjusted R2 of 0.958 and 0.978 for C. argus and S. knerii, respectively (Figure 1A). The results of the Chow tests showed no significant difference in the regression models of the studied species (F value = 0.12; P value = 0.88).The percentage volume of liver by MRI was strongly correlated with the traditional HSI for both species (r = 0.917, P <0.001 for C. argus and r = 0.974, P <0.001 for S. knerii). The regression between percentage volume of liver and HSI had a high determination coefficient with adjusted determination coefficient (R2) 0.841 and 0.949 respectively for C. argus and S. knerii (Figure 1B). There was no significant difference of species among these two linear regressions (F value = 1.70; P value = 0.19).
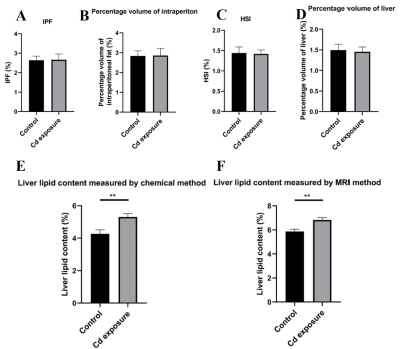
No statistically significant differences in traditional IPF and MRI percentage volume of intraperitoneal fat, traditional HSI, MRI percentage volume of liver were observed between control and Cd exposure groups (IPF: P value = 0.94; percentage volume of intraperitoneal fat: P value = 0.96; HSI: P value = 0.90; percentage volume of liver: P value = 0.83) (Figure 2A, 2B, 2C and 2D). However, liver lipid content measured by both MRI and traditional chemical methods differed significantly between control and Cd exposure groups (Figure 2E and 2F) (liver lipid content measred by chemical method: P value < 0.005; liver lipid content measured by MRI method: P value < 0.002).
Conclusions
In this study, a novel magnetic resonance imaging (MRI) approach for quantifying lipid accumulation (intraperitoneal adipose tissue and liver) has been developed and compared with conventional methods in two represented live fish species (C. argus and S. knerii). The validation and application of the present MRI method were performed with a Cd exposure experiment in C. argus. We thus believe that this MRI method is suitable for investigating the time-dependent manners of contamination on fish lipid accumulation (particularly endangered or high-economic value fish)[5], and we encourage researchers to develop and test its applicability in a range of animal models and contexts.Acknowledgements
We thank for Weiyin Vivian Liu (GE Healthcare, Beijing) for providing support.References
1. Bommuraj, V., et al., 2021. Concentration-and time-dependent toxicity of commonly encountered pesticides and pesticide mixtures to honeybees (Apis mellifera L.). Chemosphere. 266, 128974.
2.Deng, Y., et al., 2019. Spatial distribution and risk assessment of heavy metals in contaminated paddy fields - A case study in Xiangtan City, southern China. Ecotoxicol Environ Saf. 171, 281-289.
3.Guo, W., et al., 2020. Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate Affects Lipid Metabolism in Zebrafish Larvae via DNA Methylation Modification. Environmental Science & Technology. 54, 355-363.
4.Khieokhajonkhet, A., et al., 2019. Lipid distribution patterns of nine commercial fish in Thailand. Aquaculture Research. 50, 1348-1360.
5.Lu, L., et al., 2020. High-Quality Genome Assembly and Annotation of the Big-Eye Mandarin Fish (Siniperca knerii). G3 (Bethesda). 10, 877-880.
Figures