3751
Dynamic MR Imaging-guided Photothermal Therapy of Solid Tumor and Monitoring of Vascular Invasion1Department of Radiology, Affiliated Hospital of Qingdao University, Qingdao, China
Synopsis
Keywords: Molecular Imaging, DSC & DCE Perfusion, Magnetic resonance angiography
Timely evaluating the abundance of vessels and infiltration by noninvasive ways together with synchronous photothermal therapy (PTT) still faces great challenges. A multifunctional and biocompatible nanoprobes (NaBiF4: Gd@PDA@PEG) with high longitudinal relaxation rate and photothermal conversion efficiency was designed as promising photosensitizer for tumor PTT and contrast agent for MR imaging. The anatomic decrease and thinning of tumor blood vessels could be observed in real-time using noninvasive MR angiography ( TRICKS and DSC-PWI) during PTT. We demonstrated that the vascular invasion status of tumor during PTT can be monitored by utilizing our multifunctional nanoprobes, which provide possibility for clinical transformation.Introduction
Tumor neovascularization is closely related to metastasis, invasion and tumor recurrence. The quantification of new vessels in malignant solid tumors is considered to be an important independent prognostic marker1. Photothermal therapy (PTT) is a therapeutic strategy based on the absorption of NIR radiation and conversion into heat by upconversion nanoparicles2. We expected that vascular abundance and invasion status of tumor vessels can be detected noninvasively during synchronous photothermal therapy (PPT) for subcutaneous glioma, which is good for timely evaluating the efficacy of PPT for glioma.Methods
The nanoparticles NaBiF4: Gd@PDA@PEG were co incubated with C6 cells and then irradiated with NIR. A series of vitro experiments were conducted, including differentiation between living cells and dead cells by fluorescence microscope, uptake of nanoparticles by tumor cells detected by confocal laser scanning microscope(CLSM), proportion of cellular apoptosis and necrosis detected by flow cytometer( FCM), activity and toxicity detected CCK8 test. In vivo experiment, rats were injected with nanoparticles NaBiF4: Gd@PDA@PEG intravenously and NIR irradiation, we Implemented multi-modal MR angiography in vivo of rats, including 3D-FSPGR, TRICKs-MRA and DSC-PWI. TUNEL test and HE staining were performed on the glioma tissueswas to analyze the apoptosis at different stages after PTT. Changes of vascular proliferation markers, inflammatory factors and CD8 molecular were detected by immunofluorescence.Results
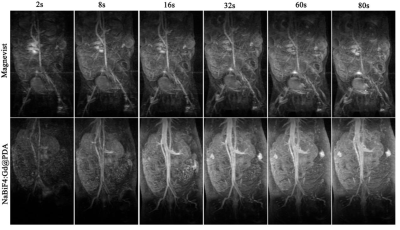
CLSM found that C6 could engulf the nanoparticles into the cytoplasm. FCM of Annexin-V FIT/PI double staining and CCK8 test showed that the proportion of necrosis and apoptosis of C6 cells increased after adding nanoparticles and NIR irradiation. Cells were only exposed to nanoparticles without NIR irradiation, the CCK8 activity assay showed that the activity of cells was almost unchanged. Figure 1a, FSPGR indicated surface of tumor after PTT obvious scab and necrosis with the decrease of the solid components of the tumor. Figure 1b, TRICKS figures showed the neovascularization of the tumor and the internal vascular network were thinning and finally disappeared after PTT. Figure 1c, parameters (rBV, rBF and MTT, TTP) derived from DSC-PWI significantly decreased. It was revealed in the Figure 3 that the imaging quality of TRICKS based on nanoprobes was better than that of commercial Magnevist because of the higher r1 relaxation from nanoprobes. In addition, Figure 2a, the TUNEL apoptosis assay found that level of tumor cell apoptosis increased. HE staining showed that tumor cells and blood vessels were significantly reduced after PTT treatment. By pathological immunofluorescence, vascular proliferation related factors (MMP-2, EphA2, MMP-9, VEGF-A, VE-cadheri) decreased, but various inflammatory factors (TNF-α, CKCL1/KC, IL-6) and CD8 molecular remarkably increased.Discussion
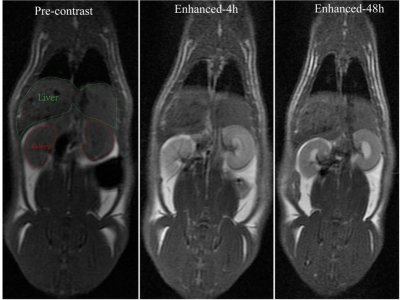
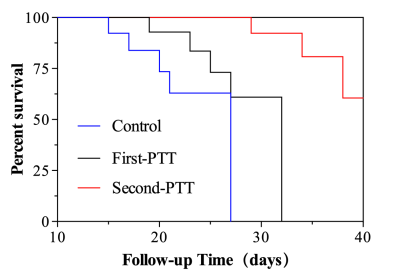
The results of Annexin-V FIT/PI double staining and CCK8 test showed that PTT treatment could cause apoptosis and necrosis of glioma cells in vitro. The results of TRICKS and DSC-PWI showed that the photothermal conversion based on nanoparticles could destroy and kill the neovascularization and internal vascular network of glioma. In conclusion, nanoparticle-mediated PTT directly double thermal ablation of tumor cells and supportive vessels. This was also confirmed by HE staining of glioma pathological tissues. In addition, the results of cck8 without irradition and HE staining on various organs (Figure 2b) showed that the toxicity of the uptake of nanoparticles to cells, tissues and organs was almost negligible in vitro and in vivo. In our study, the nanoprobe takes the kidney as the main metabolic pathway (Figure 4) and prolong survival after PTT(figure 5).Conclusion
We can ablate solid tumor and clearly monitor variation of tumorous vascular invasion status during PTT by utilizing NaBiF4: Gd@PDA@PEG. It can effectively evaluate the antivascular efficacy of PPT in the clinic treatment of glioma. Importantly, the nanoprobes presented negligible toxicity that can excrete in vivo through the kidney in time, possessing the good clinical transformation potential.Acknowledgements
This work is supported by National Natural Science Foundation of China (82001886,82272061) and Qingdao TCM Science and Technology Project(2021-zyyq21).References
- S. Yang, C. Chen, Y. Qiu, et al. Paying Attention to Tumor Blood Vessels: Cancer Phototherapy Assisted with Nano Delivery Strategies. Biomaterials. 2021;268:120562.
- W. Gao, S. Li, Z. Liu, et al. Targeting and destroying tumor vasculature with a near-infrared laser-activated “nanobomb” for efficient tumor ablation, Biomaterials. 2017;139:1–11.
Figures

Figure 1. Multimodal MR imaging of tumor at different stage of PTT.a) Enhancement degree of tumor active and necrotic area (white *) during different stage of PTT.b) White red* represents fiber crusting. Imaging tumor vascular changes via MRA-TRICKs (white arrow, new-born vessel and red arrow, right common iliac artery) in tumor-bearing rats during PTT.c) rBV, rBF and MTT, TTP maps derived from enhanced DSC-PWI in different stage of PTT.
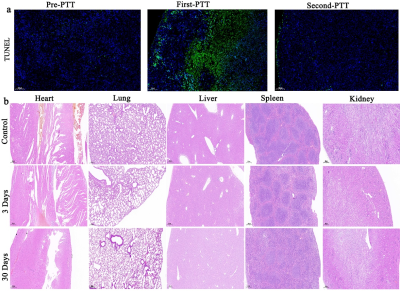
Figure 2. a) Apoptosis of tumor tissues in different treatment phases displayed on TUNEL. b)HE-stained tissues to supervise the histological changes of main organs in different time points after the intravenous injection of nanoprobes and receiving saline injection as control.