3750
MR Molecular Imaging of Pancreatic Cancer Reveals Differences in Tumor Microenvironment during Therapeutic Monitoring with Immunotherapy
Victoria Laney1 and Zheng-Rong Lu1
1Case Western Reserve University, Cleveland, OH, United States
1Case Western Reserve University, Cleveland, OH, United States
Synopsis
Keywords: Molecular Imaging, Cancer
PDAC is a highly aggressive malignant cancer and the 3rd leading cause of cancer related deaths in the US. The aggressive nature of PDAC is in part due to the desmoplastic tumor microenvironment, which promotes rapid tumor growth and impedes therapeutic efficacy of conventional therapies. Immune checkpoint inhibitors have showed specific efficacy in PDAC promotes extracellular remodeling for effective treatment of aggressive tumors. In this study we investigated the ability of an MR molecular imaging technology for image guidance and therapeutic monitoring of PD-L1 treated PDAC tumors.INTRODUCTION.Pancreatic ductal adenocarcinoma (PDAC) is a highly metastatic form of cancer, over 80% of the cases are diagnosed at advanced stages with extensive metastasis to distant organs (1). Chemotherapy is the standard care for advanced PDAC but yields limited efficacy and fails to improve the overall survival of patients. Immunotherapies using monoclonal antibodies (mAb) against immune checkpoint receptors (ICRs) such as PD-1 and CTLA4 have become breakthrough therapies for late-stage cancers (2-4). However, immunotherapies such as immune checkpoint therapy (ICT) that involve CTLA-4 and PD-L1 blockade have been established as an effective form of therapy in metastatic cancers. Despite these promising findings patients may not experience a clinical benefit with checkpoint blockade, with only 30% of patients having a positive outcome in melanoma (5). In PDAC, the heterogenous and desmoplastic nature of tumors has a suppressive effect on antitumor T cell immunity, which results in resistance to PD-L1 therapy. Thus far, imaging of the tumor immune microenvironment has been limited. Through magnetic resonance molecular imaging (MRMI) with a contrast agent specific to an alternative splice of fibronectin in the tumor microenvironment we were able to noninvasively characterize changes in the stroma of the tumor in PD-L1 treated mice. METHODS.Murine KPC-8484 PDAC cells were orthotopically implanted in C57BL/6 mice for in vivo imaging. Ten days after tumor implanted, T1-weighted MR images were obtained using a 2D fast spin-echo sequence and a 3D-FLASH sequence pre- and post-i.v.-injection of 0.1 mmol/kg MT218 using a 3T MRSolutions small animal scanner. Mice then received 3 weekly doses of PD-L1 at 150ug for a total of 12 doses. MRMI was conducted during and after treatment at weeks 4 (after 6 doses) and week 6 (after 12 doses). Contrast-to-noise ratio (CNR) were calculated using muscle as the control tissue. Differences in signal intensity was calculated by comparing pre-contrast and post-contrast images. All animal studies were conducted in accordance with CWRU’s Institutional Animal Care and Use Committee. The targeted contrast agent, MT218, was provided by Molecular Theranostics, LLC. Mice were sacrificed and tumors were excised and embedded in formalin. Fixed samples were subsequently stained for H&E, immunochemistry using G4 and PD-L1 antibody, and trichrome stain RGB for morphology and expression. RESULTS. Mice treated with PD-L1 showed increased signal intensity post-contrast injection compared to PBS treated control groups. At days 27 and day 45 PD-L1 tumors had a 2.2- and 2.0-fold increase in signal compared to control groups. Additionally, immunohistochemistry correlated with increased signal showing higher expression of EDB-FN, indicating that PD-L1 treatment causes stromal changes and increased fibronectin production (data not shown). Additionally, tumor growth is significantly different between the control group and the PD-L1 group, where the PD-L1 treated group has substantially smaller tumors than those treated with controls, despite ultimately resulting in low survival. PD-L1 mice had slightly improved survival however, 60% of mice experience spontaneous hemorrhage as a result of tumor growth. CONCLUSION.PD-L1 therapy resulted in increased fibronectin production thus allowing for a positive correlation between MRMI signal and therapeutic efficacy.
Acknowledgements
No acknowledgement found.References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. Epub 2021/01/13. doi: 10.3322/caac.21654. PubMed PMID: 33433946.2. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467-77. Epub 2008/05/27. doi: nri232610.1038/nri2326. PubMed PMID: 18500231.3. Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227-42. Epub 2013/03/09. doi: nri340510.1038/nri3405. PubMed PMID: 23470321; PMCID: 3786574.4. Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2022;19(1):37-50. Epub 2021/09/29. doi: 10.1038/s41571-021-00552-7. PubMed PMID: 34580473.5. Rawla, P. (2019). Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors, 10(1), 10–27.6. Wei, K., & Hackert, T. (2021). Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers, 13(8), 1971. https://doi.org/10.3390/cancers130819717. Hall, B. R., Cannon, A., Atri, P., Wichman, C. S., Smith, L. M., Ganti, A. K., … Batra, S. K. (2018). Advanced pancreatic cancer: a meta-analysis of clinical trials over thirty years. Oncotarget, 9(27), 19396–19405. https://doi.org/10.18632/oncotarget.250368. Zeng, S., Pöttler, M., Lan, B., Grützmann, R., Pilarsky, C., & Yang, H. (2019). Chemoresistance in Pancreatic Cancer. International Journal of Molecular Sciences, 20(18), 4504. https://doi.org/10.3390/ijms201845049. Hall, B. R., Cannon, A., Atri, P., Wichman, C. S., Smith, L. M., Ganti, A. K., … Batra, S. K. (2018). Advanced pancreatic cancer: a meta-analysis of clinical trials over thirty years. Oncotarget, 9(27), 19396–19405. https://doi.org/10.18632/oncotarget.2503610. Porter, R. L., Magnus, N. K. C., Thapar, V., Morris, R., Szabolcs, A., Neyaz, A., … Neyaz, A. (2020). Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma, 117(3). https://doi.org/10.1073/pnas.192246911711. Takahashi, K., & Ehata, S. (2018). Pancreatic tumor microenvironment confers highly malignant properties on pancreatic cancer cells. Oncogene, 2757–2772. https://doi.org/10.1038/s41388-018-0144-012. Vaidya, A., Wang, H., Qian, V., Gilmore, H., & Lu, Z. (2020). Overexpression of Extradomain-B Fibronectin is Associated with Invasion of Breast Cancer Cells, 1–19.13. Liu, Y., Zhu, S., Wang, X., Deng, J., Li, W., Zhang, P., & Liu, B. (2017). MiR-200c regulates tumor growth and chemosensitivity to cisplatin in osteosarcoma by targeting AKT2. Scientific Reports, (September), 1–9. https://doi.org/10.1038/s41598-017-14088-314. Schilb, A. L., Ayat, N. R., Vaidya, A. M., Hertz, L. M., Hall, R. C., Scheidt, J. H., … Lu, Z. (2021). Efficacy of Targeted ECO / miR-200c Nanoparticles for Modulating Tumor Microenvironment and Treating Triple Negative Breast Cancer as Non-invasively Monitored by MR Molecular Imaging, 1405–1418.15. Han, Z., & Lu, Z.-R. (2017). Targeting Fibronectin for Cancer Imaging and Therapy. Journal of Materials Chemistry. B, 5(4), 639–654. https://doi.org/10.1039/C6TB02008A16. Han, Z., Li, Y., Roelle, S., Zhou, Z., Liu, Y., Sabatelle, R., … Lu, Z.-R. (2017). Targeted Contrast Agent Specific to an Oncoprotein in Tumor Microenvironment with the Potential for Detection and Risk Stratification of Prostate Cancer with MRI. Bioconjugate Chemistry, 28(4), 1031–1040. https://doi.org/10.1021/acs.bioconjchem.6b0071917. Neri, D., & Bicknell, R. (2005). Tumour vascular targeting. Nature Reviews Cancer, 5(6), 436–446. https://doi.org/10.1038/nrc162718. Qiao, P., Ayat, N. R., Vaidya, A., Gao, S., Sun, W., Chou, S., … Lu, Z. (2020). Magnetic Resonance Molecular Imaging of Extradomain B Fibronectin Improves Imaging of Pancreatic Cancer Tumor Xenografts, 10(October), 1–13. https://doi.org/10.3389/fonc.2020.586727Figures
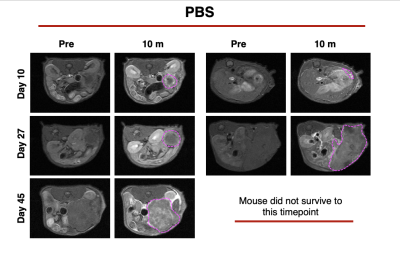
MRMI of PBS control tumors over time
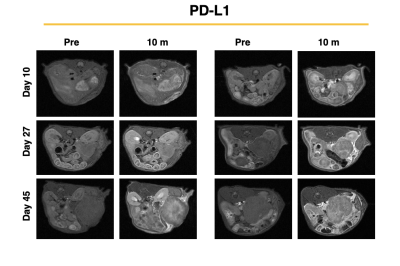
MRMI of PD-L1 treated tumors over the duration of treatment
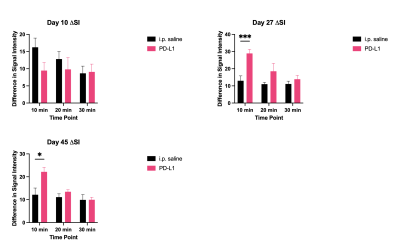
Delta signal intensity comparing control and PD-L1 treated PDAC mice at 3 imaging timepoints during the course of treatment. PD-L1 showed increased signal intensity (and CNR, not shown) at days 27 and 45 of imaging.
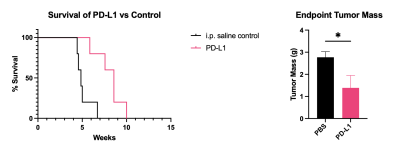
Overall survival (A) and tumor mass at terminal points (B). PD-L1 therapy improved overall survival and resulted in a reduction of tumor mass.
DOI: https://doi.org/10.58530/2023/3750