3742
A Novel MRI Platform for Long-term Stem Cell Tracking In Vivo1Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Translational Biology and Engineering Program, Ted Rogers Centre for Heart Research, Toronto, ON, Canada, 3McEwen Stem Cell Institute, University Health Network, Toronto, ON, Canada, 4Department of Biology, University of Toronto Mississauga, Mississauga, ON, Canada, 5Department of Cell and Systems Biology, University of Toronto, Toronto, ON, Canada, 6Peter Munk Cardiac Centre, University Health Network, Toronto, ON, Canada, 7Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada, 8The Edward S. Rogers Sr. Department of Electrical and Computer Engineering, University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Molecular Imaging, Cell Tracking & Reporter Genes, stem cell
To extend our previously reported bright-ferritin cell tracking platform to monitoring stem cell therapy, we investigated tracking human embryonic stem cells transplanted intramuscularly in immune-compromised mice. With this technology, the stem cells impart a bright, T1-induced contrast. In-vitro assays of viability and
Introduction
Stem-cell therapy is a promising mode of treatment to recover the function of damaged tissues through delivering therapeutic cells to the site of injury. It is especially relevant in the case of tissues with low regenerative capacities, such as the brain and heart 1. Currently, when stem cells are injected into the body, there is no information on their survival, growth, and contribution to improved tissue function until histology is performed. While this approach can be adopted in animal studies, it, nonetheless, precludes probing cell viability, retention, distribution, and interaction with host tissue in a temporal manner. A technology that enables non-invasive cell tracking in deep tissue would address these hurdles and pave the way for stem cell treatment optimization and translation. To accomplish this, here we validate and demonstrate a novel MRI cell tracking technology, one that provides longitudinal and “on-demand” signal recall, to monitor transplanted human embryonic stem cells in rodents.Methods
Human embryonic stem cells (hESCs) ESI-017 were genetically modified to stably overexpress human ferritin using CRISPR- Cas9 system 2. The cellular toxicity associated with ferritin overexpression and MR-active metal ion supplementation was assessed based on cell viability, proliferation, and metabolic activity. The genetically modified hESCs were characterized based on stem cell pluripotency. Labeled cells were supplemented with MnCl2 and imaged in vitro as cell pellets on a preclinical 3T MR scanner (MR Solutions, Guildford, UK). T1-weighted images and T1 relaxation times were analyzed to assess the contrast signals. For the in-vivo study, three million cells were injected into the leg muscle of NOD-SCID mice, with ferritin-overexpressing cells in one leg and wild-type cells in the contralateral leg. MnCl2 were administrated subcutaneously. T1-weighted fast spin echo and T1 mapping 3 were performed on mice on the 3T scanner to assess cell survival, proliferation, and teratoma formation. Histological analysis was used to validate MRI results.Results and Discussion
We generated a mutant hESC cell line that stably overexpresses human ferritin using a non-viral CRISPR-Cas 9 system 2, showing a higher protein expression level of ferritin than wild-type cells (Figure 1). Both wild-type and ferritin-overexpressing hESCs were dosed with different concentrations of MnCl2; the critical dosing point that did not affect cell viability, proliferation, and metabolic activity was 0.1 mM for 24 hr (Figure 2). Immunostaining for the pluripotency markers OCT4 and SSEA4 confirmed that gene editing and MnCl2supplementation did not affect the “stemness” of the cells (Figure 3). Using the critical dosing point, we obtained T1-induced bright contrast from ferritin-overexpressing hESCs in vitro (Figure 4a). At baseline, wild-type cells and ferritin-overexpressing cells displayed similar contrasts level. Upon MnCl2 supplementation, ferritin-overexpressing cells displayed a T1 that was 1.7-fold lower than wild-type cells (Figure 4b). In-vivo bright-contrast efficiency for cell tracking is shown in Figure 5. At baseline (Day 0), there was no T1-induced contrast in either leg. After MnCl2 administration (Day 1), bright contrast appeared at the cell injection sites and persisted for up to five days. Injected cells grew into teratoma after eight weeks, and histology confirmed its origin from the injected human cells (Figure 5).Conclusion
Our novel MRI-based cell tracking technology enabled long-term and on-demand tracking of human embryonic stem cells after transplantation into the mouse leg muscle. With the ability to “see” the cells in living bodies, this technology will serve as a valuable imaging tool to assess and improve individual platforms for stem cell therapy or understanding the involvement of cells in disease.Acknowledgements
No acknowledgement found.References
1. Naumova AV, Modo M, Moore A, Murry CE, Frank JA. Clinical imaging in regenerative medicine. Nat Biotechnol. 2014;32(8):804-818. doi:10.1038/nbt.2993
2. Szulc DA, Lee XA, Cheng HM, Cheng HM. Bright Ferritin-a Reporter Gene Platform for On-Demand, Longitudinal Cell Tracking on MRI. iScience. 2020;23(8):101350. doi: 10.1016/j.isci.2020.101350
3. Cheng HL, Wright GA. Rapid high-resolution T(1) mapping by variable flip angles: accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med 2006;55(3):566-74.
Figures
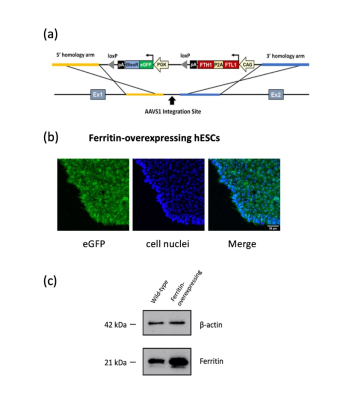
Figure 1. CRISPR/Cas9-engineered hESCs demonstrate stable ferritin overexpression.
(a) Schematic of the plasmid vector to insert human ferritin gene into the AAVS1 locus in the genome of hESCs ESI-017 using a non-viral CRISPR/Cas9 system. (b) Immunofluorescence imaging confirmed gene insertion with eGFP tag (green). Cell nuclei were stained by Hoeschst 33342 (blue). Scale bar = 50 μm. (c) Western blots confirmed ferritin overexpression in hESCs. Beta-actin was used for loading control.
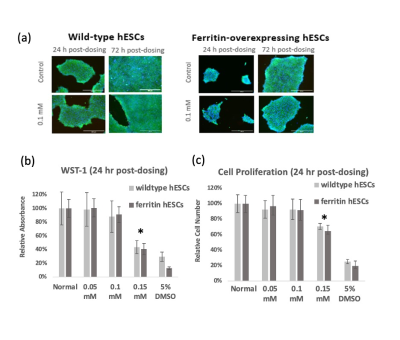
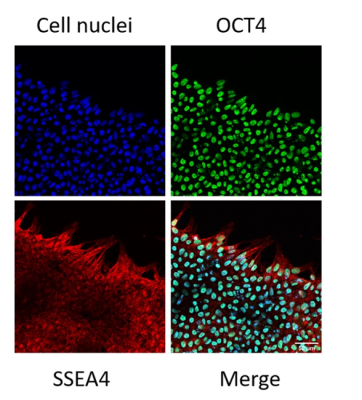
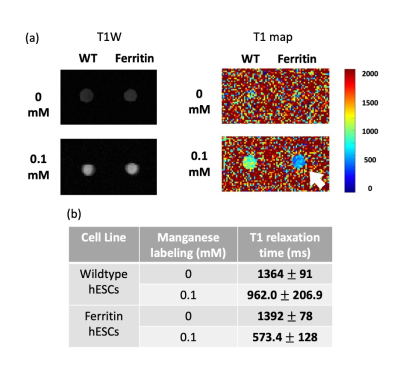
Figure 4. Bright-contrast efficiency of ferritin-overexpressing hESCs demonstrated by in-vitro MRI. (a) T1-weighted spin echo image and T1 map of wild-type (WT) and ferritin-overexpressing (Ferritin) hESCs supplemented with 0.1 mM Mn for 24 hr. (b) Mean T1 values ± standard deviations of each cell pellet shown in (a).
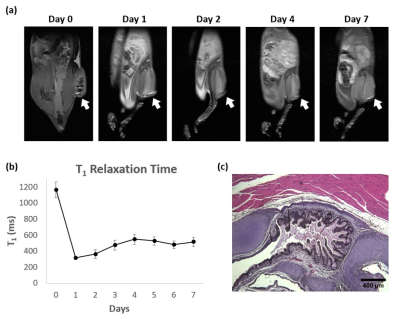
Figure 5. In-vivo MRI of hESC Cell Injections in Mice. (a) T1w FSE images of a representative NOD/SCID mouse injected with ferritin-overexpressing cells in the right leg and wild-type cells in the contralateral leg. 24 hr after MnCl2 administration (Day 1), the ferritin-overexpressing hESCs injection site (indicated by white arrow) exhibited significant bright contrast. (b) Quantitative T1 maps showed that T1 values of cell injection site reduced significantly after MnCl2 administration and was lower than baseline for seven days. (c) H&E stain for teratoma formed after 8 weeks.