3741
manganese-based nanosystem for dual-modality imaging guided STING pathway with immunotherapy against triple-negative breast cancer1Department of Radiology, First Affiliated Hospital of Jinan University, Guangzhou, China, 2Guangzhou Medical University, Guangzhou, China, 3School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou, China, 4Department of Radiology, Guangzhou First People’s Hospital, Guangzhou, China
Synopsis
Keywords: Molecular Imaging, Cancer, tumor, dual-modality imaging, STING pathway, Immunotherapy
Due to the high incidence and mortality rate, breast cancer has become the major cause of cancer death among females. This study reports a kind of functional nanocomposite, which was designed to comprises Pluronic F127 to self-assembly manganese carbonyl (MnCO) and IR780 dyes into one system, exhibiting NIR-II and MR dual-modal imaging guided STING pathway and anti-tumor immunity against triple-negative breast cancer.INTRODUCTION
Breast cancer has become the tumor with highest incidence in the world, and it is an urgent to develop new diagnostic and therapeutic approaches to treat breast cancer[1]. This nanocomposite we manufactured could diagnose the tumor and confirm the time window of laser radiation via dual-modal imaging, and effectively suppress tumor growth and metastasis through phototherapy, STING pathway and anti-tumor immunity.METHODS
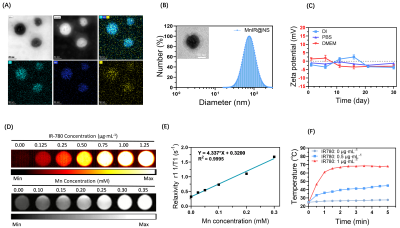
Preparation and characterization of MnCO based nanocomposite (MnIR@NS)Firstly, the MnCO based nanosystem (MnIR@NS) was obtained though ultrasonic self-assembly towards Pluronic F127, MnCO and IR780 dyes into one system. The site, morphology and the zeta potential of Mn@IR was characterized by DLS and TEM[2]. Secondly, the NIR imaging and MR-T1 weight imaging property of MnIR@NS obained by second near-infrared imager and 3.0 T MRI system respectively[3]. To evaluate the photothermal effect, MnIR@NS of different concentrations were exposed to continuous laser for 5 minutes[4].
Biocompatibility, phototherapy and macrophage polarization in vitro
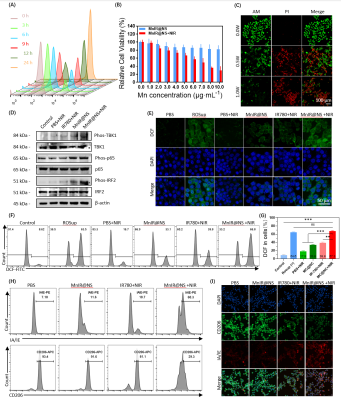
The uptake level of MnIR@NS at various time points in 4T1 cells was assessed by flow cytometry (FCM). The photothermal effect of MnIR@NS at the cellular level was proceeded by CCK-8 assay and propidium iodide (PI) and calcien-AM staining. The intracellular expression levels of characteristic proteins downstream of the STING pathway towards 4T1 cells with different treatments were measured by western blot[5]. The ROS level towards 4T1 cells with different treatments was measured by DCF staining which was observed by laser confocal microscope and FCM. To investigate the ability of MnIR@NS to repolarize M2 macrophages to M1 phenotype, M1 (F4/80+IA/IE+) and M2 (F4/80+CD206+) in RAW264.7 cells with various treatment were estimated by flow cytometry[6].
MRI-NIRF dual-modality imaging in vivo
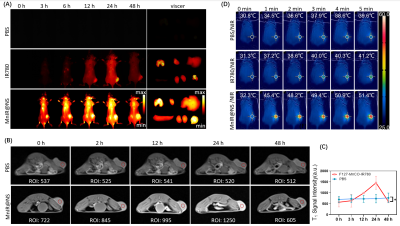
Breast cancer model was established via Balb/c mouse (female, 6 weeks old) injected 4T1 cells. When the tumor volume reached to 800 mm3 approximately, the tumor-bearing mice were randomly divided into three groups (PBS, free IR780 and MnIR@NS, n=3/group) and underwent NIRF II imaging. MR imaging in vivo of two groups mice (PBS group and MnIR@NS group, n=3/group) was performed on a 3.0T MR system (Siemens, Gremany). The T1-weighted images of tumors were obtained before injection and post injection (0 h, 2 h, 12 h, 24 h, 48 h). Parameters of T1-weighted imaging were as follow: FOV: 120 mm, TR/TE: 720/9.4 ms, slice thickness: 2 mm and slice spacing: 2 mm.
Anti-tumor Immunity synergistic therapy in vivo
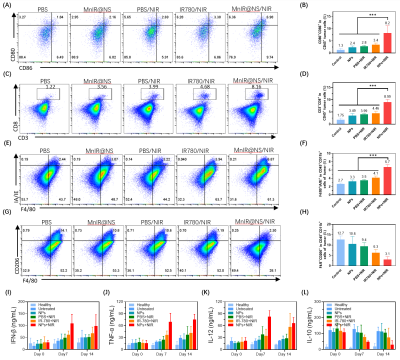
Firstly, the mice were classified as six groups randomly (n=5) as the tumors grew to approximately 100 mm3: ①health (no tumor), ②PBS, ③MnIR@NS, ④PBS+808 nm NIR, ⑤IR780+808 nm NIR, ⑥MnIR@NS+808 nm NIR. At 24 h post-injection with PBS, IR780 and MnIR@NS, mice of group ④⑤⑥ were irradiated with 808 nm laser (1.0 W·cm-2, 5 min). Secondly, dendritic cell (DCs) (CD45+CD11c+CD80+CD86+), cytotoxic T cells (CTLs) T cells(CD45+CD3+CD8+), M1 (CD45+F4/80+IA/IE+) and M2 (CD45+F4/80+CD206+) macrophages in tumor of mice with different treatments at 7 days. In addition, the level of IFN-β, TNF-γ, IL-12 and IL-10 were measured by enzyme-linked immunosorbent assay.
After undergoing different treatments, tumor volume and mouse body weight were measured every two days. And the tumors and vital organs were collected at 14th days and stained with hematoxylin-eosin, Ki67 and tunnel.
All statistical analyses were performed using the software GraphPad Prism 8.0 (*p < 0.05, **p < 0.01).
RESULTS AND DISCUSSIONS
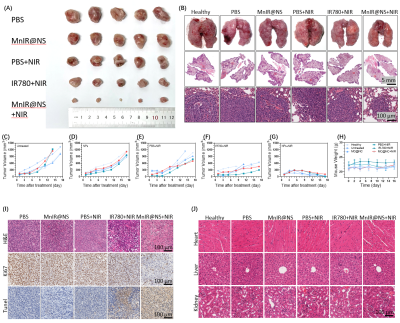
Firstly, MnIR@NS showed a diameter of 79.99±3.06 nm and the TEM images further confirmed the core-shell spherical shape (Fig. 1A-C), which showed great potential for tumor accumulation through the EPR effect. Secondly, excellent cellular uptake capacity and photothermic effect in 4T1 cells was in Fig. 2A-C. Furthermore, remarkable NIR II and MRI imaging potential of MnIR@NS were showed in vitro (Fig. 1D&E) and in vivo (Fig. 3A-C). What's more, the excellent photothermal effect of MnIR@NS based laser irradiation were demonstrated in vitro (Fig 1F) and in vivo (Fig. 3D). STING pathway was activated (Fig. 2D) and the generation of ROS by MnIR@NS based laser irradiation were identified (Figs. 2E-G). Together, all of these activated immunotherapy because of MnIR@NS based phototherapy[7], and the antitumor immune cells (DCs, CD8+ CLT cells and M1 macrophages) were upregulated along with the protumor immune cells (M2 macrophages) were downregulated (Fig. 2H-I & 4A-H)[8, 9]. Meanwhile, The level of anti-tumor cytokines (IFN-β, TNF-γ, IL-12) was increased and promo-tumor cytokines (IL-10) were decreased (Fig. 4I-L)[10]. Finally, compared with the control groups, primary tumors and lung metastases in MnIR@NS+NIR group shrank significantly (Fig. 5A-J).CONCLUSION
We developed a multifunctional MnIR@NS nanocomposite, which could diagnose the tumor and confirm the time window of treatment via NIR II/MRI dual-modal imaging, and effectively suppress tumor growth thought phototherapy and anti-tumor immunity. This MnCO-based nanosystem provided a promising strategy for cancer theranostic applications.Acknowledgements
This study was funded by the National Natural Science Foundation of China (81971574, 82271938), the Natural Science Foundation of Guangdong Province (2021A1515011350, the GuangDong Basic and Applied Basic Research Foundation (2021A1515220060), the Science and Technology Project of Guangzhou (202102010025), the Special Fund for the Construction of High-level Key Clinical Specialty (Medical Imaging) in Guangzhou, Guangzhou Key Laboratory of Molecular Imaging and Clinical Translational Medicine (202201020376).References
[1]. Sung, H., et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians, 2021. 71(3): p. 209-249.
[2]. Wu, D., et al., Mesoporous Polydopamine Carrying Manganese Carbonyl Responds to Tumor Microenvironment for Multimodal Imaging‐Guided Cancer Therapy. Advanced functional materials, 2019. 29(16): p. 1900095-n/a.
[3]. Guan, X., et al., Iron oxide-based enzyme mimic nanocomposite for dual-modality imaging guided chemical phototherapy and anti-tumor immunity against immune cold triple-negative breast cancer. Chemical engineering journal (Lausanne, Switzerland : 1996), 2021. 425: p. 130579.
[4]. Peng, J., et al., Photosensitizer Micelles Together with IDO Inhibitor Enhance Cancer Photothermal Therapy and Immunotherapy. Adv Sci (Weinh), 2018. 5(5): p. 1700891.
[5]. Sun, X., et al., Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nature nanotechnology, 2021. 16(11): p. 1260-1270.
[6]. Chang, M., et al., Colorectal Tumor Microenvironment‐Activated Bio‐Decomposable and Metabolizable Cu2O@CaCO3 Nanocomposites for Synergistic Oncotherapy. Advanced materials (Weinheim), 2020. 32(43): p. n/a-n/a.
[7]. Chu, K.F. and D.E. Dupuy, Thermal ablation of tumours: biological mechanisms and advances in therapy. Nature reviews. Cancer, 2014. 14(3): p. 199-208.
[8]. Shengjun Xu, S.L.Q.S., Self-Activated Cascade-Responsive Sorafenib and USP22 shRNA Co-Delivery System for Synergetic Hepatocellular Carcinoma Therapy. Advanced Science, 2021. 2003042(8): p. 1-15.
[9]. Yan, J., et al., Engineering Radiosensitizer-Based Metal-Phenolic Networks Potentiate STING Pathway Activation for Advanced Radiotherapy. Adv Mater, 2022. 34(10): p. e2105783.
[10]. Li, X., et al., Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat Nanotechnol, 2022. 17(8): p. 891-899.
Figures