3700
Ex-vivo MR spectroscopy in paraformaldehyde-fixed mouse brain
Alireza Abaei1, Dinesh K Deelchand 2, Stefano Antonucci3, Jelena Scekic-Zahirovic3, Florian olde Heuvel3, Francesco Roselli3, and Volker Rasche4
1Ulm University, Ulm, Germany, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3German Center for Neurodegenerative Diseases (DZNE), Ulm, Germany, 4Core Facility Small Animal Imaging (CF-SANI), University of Ulm, Ulm, Germany
1Ulm University, Ulm, Germany, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3German Center for Neurodegenerative Diseases (DZNE), Ulm, Germany, 4Core Facility Small Animal Imaging (CF-SANI), University of Ulm, Ulm, Germany
Synopsis
Keywords: Spectroscopy, Spectroscopy, in vivo ex vivo MRS
The goal of this study is to demonstrate the feasibility and reliability of metabolite characterization by MR spectroscopy ex vivo in order to maximize the information obtained from a single brain specimen, without the limitations such as scanning time, degraded MRI quality due to intervening tissue and motion artifacts. We demonstrate that ex-vivo spectroscopy obtained after rapid PBS/PFA perfusion is strongly correlated with the MR spectrum previously acquired in the same animal but in vivo but distinct abnormalities due to hypoxia and incomplete fixation do appear; the quality of the spectrum quickly degrades upon longer fixation times.Purpose:
Current methods for metabolomic fingerprinting by MR spectroscopy are limited by the need to have a sufficient signal-to-noise ratio, achieved either by employing large volumes of interest or by involving long acquisition times. Both approaches constrain either the biological relevance of the measure, if it is acquired from a volume encompassing different, not homogeneous structures, or the quality of the spectrum and the number of metabolites that can be reliably measured. An ideal method would require the maximization of the S/N by removing all intervening structures and place the dissected brain in close vicinity of the detection coil; however, this would require an approach to freeze the metabolite concentrations so that no post-mortem degradation occurs. Since Paraformaldehyde is commonly used as histological fixative, it may be sufficient to prevent metabolite degradation and at the same time generate samples that can be later used for histological or immunohistolgical procedures. This approach would therefore maximize the information retrieved by a single brain sample, allowing the decrease in the number of animals used in research.Methods:
C57/B6 mice were transcardially perfused with PBS (1.5 ml/g) and then with PFA (4% in PBS, 1.5 ml/g); the brain was extracted and placed in Fluorinert at 4°C. After the first ex-vivo acquisition, the brain was post-fixed in PFA (4%) for 18h, washed in PBS and then once again stored in Fluorinert at 4°C.Experiments were performed on a dedicated ultrahigh field 11.7T small animal system (BioSpec 117/16, Bruker Biospin, Ettlingen, Germany) equipped with a 9 cm gradient insert (BGA-S9) operating with ParaVision 6.01. All data were acquired using a cryogenically cooled 1H two-element surface transmit/receive coil (MRI CryoProbe™, Bruker BioSpec, Ettlingen, Germany). A home-built head restrainer was used to properly immobilize the animal's head during in vivo session, ensuring stability and reproducibility of the experimental setup. For ex-vivo measurements, 15 ml tube containing the brain, placed directly inside the probe head. Volume-of-interest (VOI) was planned based on T1-weighted multi-slice FLASH (TR/TE=193/5ms, FA=17.5°) images. Field homogeneity was adjusted using MAPSHIM. LASER (1) (TR/TE=5000/16.75ms, averages=768) combined with VAPOR water suppression was used (2). 1H MR spectra were acquired from 15 mm3 (=1.4 x 3 x 3.6 mm3) volume located in the caudoputamen of adult female C57BL/6 both in vivo and ex-vivo brain. Single-shot data were frequency and phase corrected prior to summation (3). Unsuppressed water signal was used as an internal reference as well as for eddy current, zero-order and first-order phase correction.Results:
We have acquired MRS spectra in vivo and soon after perfusion fixation with PFA 4%. The stability of the spectra recorded during the in vivo scanning (considering the bins averaged per minute over the whole acquisition period) is substantially larger in the in vivo vs ex vivo recording; thus, ex-vivo spectroscopy allows a superior signal-to-noise ratio (Figure 1). The comparison of the in vivo and ex-vivo spectra reveals substantial changes occurring after perfusion-fixation (Figure 2). In particular, the lactate peak is increased in the ex-vivo, suggesting that metabolic activities are not fully blocked by the cooling of the brain at 4°C and by perfusing with PFA. Glutamine is increased in the ex vivo, but glutamate is decreased, suggesting that although neuronal physiology may be arrested, metabolism in astrocytes may still be ongoing. This alteration of metabolites even during the course of the acquisition of spectra in the perfusion-fixation stage, is manifested by an increase in mIns level while tCr decreased (Figure 3).Discussion:
We demonstrate the feasibility of ex-vivo MR spectroscopy upon fast brain cooling and 1f fixation with PFA; the signal-to-noise ratio is substantially improved in the ex-vivo recording, thanks to the removal of artifacts (hearbeat, breathing) and to the apposition of the brain to the detection coil (no intervening bone and skin). However, the fast cooling of the mouse brain with ice-cold PBS followed by PFA is not sufficient to fully stabilize the metabolite profile: the elevation in the lactate peak points toward ongoing hypoxic glycolysis and the increase in glutamine and decrease in glutamate suggest ongoing metabolic activities in astrocytes occurring during the perfusion-fixation and dissection stage. Thus, the ex-vivo spectrum does not recapitulate the in vivo spectrum and cannot be used as substitute of the latter. However, the observed changes provide a window into the preservation of the tissue upon fixation and reveal the limitations of perfusion-fixation for preserving the integrity of the tissue free of hypoxia and other post-mortem artifacts.Acknowledgements
DFG grant no. 251293561 to FR DKD acknowledges support from National Institutes of Health grants: BTRC P41 EB027061 and P30 NS076408.References
1. Garwood, M. & DelaBarre, L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 153, 155-177, (2001).
2. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med (1999) 41:649.
3. http://www.cmrr.umn.edu/downloads/mrspa
Figures
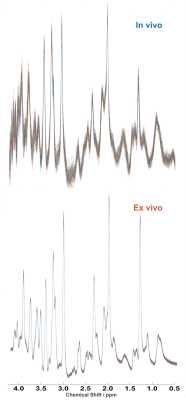
Figure 1: Stacked plot of 64 1H spectra representative of the stability of the spectra during the in vivo acquisition (considering the bins averaged per minute over the whole acquisition period) in comparison to ex vivo.
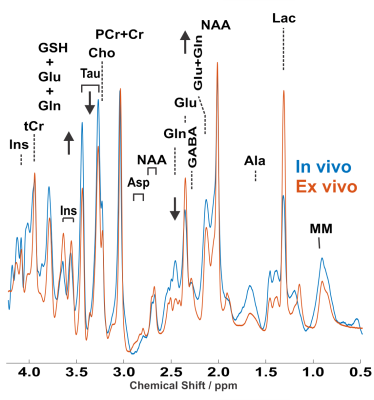
Figure 2: Representative in vivo and ex vivo 1H spectra from 15 µl volume, located in the caudoputamen of the adult female C57BL/6
mouse. LASER sequence, TR/TE: 5000/16.75 ms: 10 kHz spectral width, 4096 data
points and 768 averages. For display purposes, a Gaussian multiplication (gf = 0.12 s) was applied. Insets show FLASH images with the selected VOI
locations.
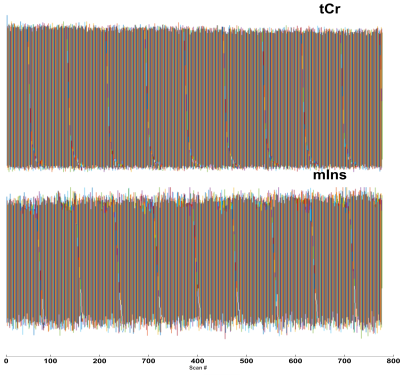
Peak height variations of tCr and mIns in 768 recorded spectra during the acquisition of spectra in the perfusion-fixation stage. an increase in mIns level and decrease tCr can be observed.
DOI: https://doi.org/10.58530/2023/3700